Genomic analyses of germline and somatic variation in high-grade serous ovarian cancer
- PMID: 37460928
- PMCID: PMC10351177
- DOI: 10.1186/s13048-023-01234-x
Genomic analyses of germline and somatic variation in high-grade serous ovarian cancer
Abstract
Background: High-grade serous ovarian cancers (HGSCs) display a high degree of complex genetic alterations. In this study, we identified germline and somatic genetic alterations in HGSC and their association with relapse-free and overall survival. Using a targeted capture of 557 genes involved in DNA damage response and PI3K/AKT/mTOR pathways, we conducted next-generation sequencing of DNA from matched blood and tumor tissue from 71 HGSC participants. In addition, we performed the OncoScan assay on tumor DNA from 61 participants to examine somatic copy number alterations (SCNA).
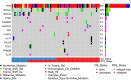
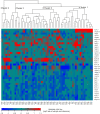
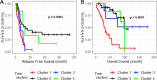
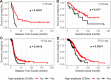
Results: Approximately one-third of tumors had loss-of-function (LOF) germline (18/71, 25.4%) or somatic (7/71, 9.9%) variants in the DNA homologous recombination repair pathway genes BRCA1, BRCA2, CHEK2, MRE11A, BLM, and PALB2. LOF germline variants also were identified in other Fanconi anemia genes and in MAPK and PI3K/AKT/mTOR pathway genes. Most tumors harbored somatic TP53 variants (65/71, 91.5%). Using the OncoScan assay on tumor DNA from 61 participants, we identified focal homozygous deletions in BRCA1, BRCA2, MAP2K4, PTEN, RB1, SLX4, STK11, CREBBP, and NF1. In total, 38% (27/71) of HGSC patients harbored pathogenic variants in DNA homologous recombination repair genes. For patients with multiple tissues from the primary debulking or from multiple surgeries, the somatic mutations were maintained with few newly acquired point mutations suggesting that tumor evolution was not through somatic mutations. There was a significant association of LOF variants in homologous recombination repair pathway genes and high-amplitude somatic copy number alterations. Using GISTIC analysis, we identified NOTCH3, ZNF536, and PIK3R2 in these regions that were significantly associated with an increase in cancer recurrence and a reduction in overall survival.
Conclusions: From 71 patients with HGCS, we performed targeted germline and tumor sequencing and provided a comprehensive analysis of these 557 genes. We identified germline and somatic genetic alterations including somatic copy number alterations and analyzed their associations with relapse-free and overall survival. This single-site long-term follow-up study provides additional information on genetic alterations related to occurrence and outcome of HGSC. Our findings suggest that targeted treatments based on both variant and SCNA profile potentially could improve relapse-free and overall survival.
Keywords: Germline mutations; High-grade serous ovarian cancer; Homologous recombination repair; Somatic mutations; Somatic copy number alterations.
© 2023. The Author(s).
Conflict of interest statement
Both Drs. Wakabayashi and Cristea now work at Regeneron; they worked at City of Hope when the data were generated. Dr. Han receives research funding from Vergent Biosciences. None of the other authors report conflicts of interest.
Figures


Update of
-
Genomic Analyses of Germline and Somatic Variation in High-Grade Serous Ovarian Cancer.Res Sq [Preprint]. 2023 Feb 20:rs.3.rs-2592107. doi: 10.21203/rs.3.rs-2592107/v1. Res Sq. 2023. Update in: J Ovarian Res. 2023 Jul 17;16(1):141. doi: 10.1186/s13048-023-01234-x. PMID: 36865331 Free PMC article. Updated. Preprint.
References
-
- Laine A, Sims TT, Le Saux O, Ray-Coquard I, Coleman RL. Treatment Perspectives for Ovarian Cancer in Europe and the United States: Initial Therapy and Platinum-Sensitive Recurrence after PARP Inhibitors or Bevacizumab Therapy. Curr Oncol Rep. 2021;23(12):148. doi: 10.1007/s11912-021-01128-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

