Treatment with GLP-1 receptor agonists is associated with significant weight loss and favorable headache outcomes in idiopathic intracranial hypertension
- PMID: 37460968
- PMCID: PMC10353241
- DOI: 10.1186/s10194-023-01631-z
Treatment with GLP-1 receptor agonists is associated with significant weight loss and favorable headache outcomes in idiopathic intracranial hypertension
Abstract
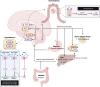
Background: In idiopathic intracranial hypertension (IIH), sustained weight loss is the main pillar in modifying disease course, whereby glucagon-like peptide-1 receptor agonists (GLP-1-RAs) could present an attractive treatment option.
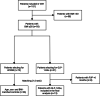
Methods: In this open-label, single-center, case-control pilot study, patients with IIH (pwIIH) and a body mass index (BMI) of ≥ 30 kg/m2 were offered to receive a GLP-1-RA (semaglutide, liraglutide) in addition to the usual care weight management (UCWM). Patients electing for UCWM only served as a control group matched for age-, sex- and BMI (1:2 ratio). The primary endpoint was the percentage weight loss at six months (M6) compared to baseline. Secondary endpoints included the rate of patients with a weight loss of ≥ 10%, monthly headache days (MHD), the rate of patients with a ≥ 30% and ≥ 50% reduction in MHD, visual outcome parameters, and adverse events (AEs).
Results: We included 39 pwIIH (mean age 33.6 years [SD 8.0], 92.3% female, median BMI 36.3 kg/m2 [IQR 31.4-38.3]), with 13 patients being treated with GLP-1-RAs. At M6, mean weight loss was significantly higher in the GLP-1-RA group (-12.0% [3.3] vs. -2.8% [4.7]; p < 0.001). Accordingly, weight loss of ≥ 10% was more common in this group (69.2% vs. 4.0%; p < 0.001). Median reduction in MHD was significantly higher in the GLP-1-RA group (-4 [-10.5, 0.5] vs. 0 [-3, 1]; p = 0.02), and the 50% responder rate was 76.9% vs. 40.0% (p = 0.04). Visual outcome parameters did not change significantly from baseline to M6. Median reduction in acetazolamide dosage was significantly higher in the GLP-1-RA group (-16.5% [-50, 0] vs. 0% [-25, 50]; p = 0.04). AEs were mild or moderate and attributed to gastrointestinal symptoms in 9/13 patients. None of the AEs led to premature treatment discontinuation.
Conclusions: This open-label, single-center pilot study suggests that GLP-1-RAs are an effective and safe treatment option for achieving significant weight loss with a favorable effect on headache, leading to reduced acetazolamide dosage in pwIIH.
Keywords: Glucagon-like peptide-1; Headache; Idiopathic intracranial hypertension; Visual worsening; Weight loss.
© 2023. The Author(s).
Conflict of interest statement
Nik Krajnc: has participated in meetings sponsored by, received speaker honoraria or travel funding from BMS/Celgene, Janssen-Cilag, Merck, Novartis, Roche and Sanofi-Genzyme and held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Bianca Itariu: declares no conflict of interest relevant to this study.
Stefan Macher: declares no conflict of interest relevant to this study.
Wolfgang Marik: declares no conflict of interest relevant to this study.
Jürgen Harreiter: has participated in meetings sponsored by, received speaker/consultancy honoraria or travel funding from Novo Nordisk, Sanofi, Novartis, Eli Lilly, Boehringer-Ingelheim. He has received unrestricted research grants from Bayer and Astra Zeneca.
Martin Michl: declares no conflict of interest relevant to this study.
Klaus Novak: declares no conflict of interest relevant to this study.
Christian Wöber: has received honoraria consultancy/speaking from Apomedica, Curelator, Eli Lilly, Grünenthal, Hermes, Lundbeck, Novartis, Pfizer, Ratiopharm/Teva, and Stada.
Berthold Pemp: declares no conflict of interest relevant to this study.
Gabriel Bsteh: has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

