Talin2 and KANK2 functionally interact to regulate microtubule dynamics, paclitaxel sensitivity and cell migration in the MDA-MB-435S melanoma cell line
- PMID: 37460977
- PMCID: PMC10353188
- DOI: 10.1186/s11658-023-00473-6
Talin2 and KANK2 functionally interact to regulate microtubule dynamics, paclitaxel sensitivity and cell migration in the MDA-MB-435S melanoma cell line
Abstract
Background: Focal adhesions (FAs) are integrin-containing, multi-protein structures that link intracellular actin to the extracellular matrix and trigger multiple signaling pathways that control cell proliferation, differentiation, survival and motility. Microtubules (MTs) are stabilized in the vicinity of FAs through interaction with the components of the cortical microtubule stabilizing complex (CMSC). KANK (KN motif and ankyrin repeat domains) family proteins within the CMSC, KANK1 or KANK2, bind talin within FAs and thus mediate actin-MT crosstalk. We previously identified in MDA-MB-435S cells, which preferentially use integrin αVβ5 for adhesion, KANK2 as a key molecule enabling the actin-MT crosstalk. KANK2 knockdown also resulted in increased sensitivity to MT poisons, paclitaxel (PTX) and vincristine and reduced migration. Here, we aimed to analyze whether KANK1 has a similar role and to distinguish which talin isoform binds KANK2.
Methods: The cell model consisted of human melanoma cell line MDA-MB-435S and stably transfected clone with decreased expression of integrin αV (3αV). For transient knockdown of talin1, talin2, KANK1 or KANK2 we used gene-specific siRNAs transfection. Using previously standardized protocol we isolated integrin adhesion complexes. SDS-PAGE and Western blot was used for protein expression analysis. The immunofluorescence analysis and live cell imaging was done using confocal microscopy. Cell migration was analyzed with Transwell Cell Culture Inserts. Statistical analysis using GraphPad Software consisted of either one-way analysis of variance (ANOVA), unpaired Student's t-test or two-way ANOVA analysis.
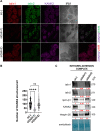
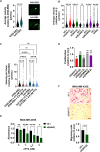
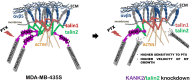
Results: We show that KANK1 is not a part of the CMSC associated with integrin αVβ5 FAs and its knockdown did not affect the velocity of MT growth or cell sensitivity to PTX. The talin2 knockdown mimicked KANK2 knockdown i.e. led to the perturbation of actin-MT crosstalk, which is indicated by the increased velocity of MT growth and increased sensitivity to PTX and also reduced migration.
Conclusion: We conclude that KANK2 functionally interacts with talin2 and that the mechanism of increased sensitivity to PTX involves changes in microtubule dynamics. These data elucidate a cell-type-specific role of talin2 and KANK2 isoforms and we propose that talin2 and KANK2 are therefore potential therapeutic targets for improved cancer therapy.
Keywords: Cortical microtubule stabilizing complex; Focal adhesion; KANK1; KANK2; Talin1; Talin2.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures



Similar articles
-
KANK2 Links αVβ5 Focal Adhesions to Microtubules and Regulates Sensitivity to Microtubule Poisons and Cell Migration.Front Cell Dev Biol. 2020 Mar 3;8:125. doi: 10.3389/fcell.2020.00125. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32195252 Free PMC article.
-
Talin-KANK1 interaction controls the recruitment of cortical microtubule stabilizing complexes to focal adhesions.Elife. 2016 Jul 13;5:e18124. doi: 10.7554/eLife.18124. Elife. 2016. PMID: 27410476 Free PMC article.
-
Force-Dependent Regulation of Talin-KANK1 Complex at Focal Adhesions.Nano Lett. 2019 Sep 11;19(9):5982-5990. doi: 10.1021/acs.nanolett.9b01732. Epub 2019 Aug 13. Nano Lett. 2019. PMID: 31389241
-
The tale of two talins - two isoforms to fine-tune integrin signalling.FEBS Lett. 2018 Jun;592(12):2108-2125. doi: 10.1002/1873-3468.13081. Epub 2018 May 18. FEBS Lett. 2018. PMID: 29723415 Free PMC article. Review.
-
KANK family proteins in cancer.Int J Biochem Cell Biol. 2021 Feb;131:105903. doi: 10.1016/j.biocel.2020.105903. Epub 2020 Dec 10. Int J Biochem Cell Biol. 2021. PMID: 33309958 Review.
Cited by
-
The Potential of Extracellular Matrix- and Integrin Adhesion Complex-Related Molecules for Prostate Cancer Biomarker Discovery.Biomedicines. 2023 Dec 28;12(1):79. doi: 10.3390/biomedicines12010079. Biomedicines. 2023. PMID: 38255186 Free PMC article. Review.
-
The extracellular matrix component perlecan/HSPG2 regulates radioresistance in prostate cancer cells.Front Cell Dev Biol. 2024 Aug 1;12:1452463. doi: 10.3389/fcell.2024.1452463. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39149513 Free PMC article.
-
Matrix Remodeling Associated Genes (MXRAs): structural diversity, functional significance, and therapeutic potential in tumor microenvironments.Discov Oncol. 2025 May 20;16(1):833. doi: 10.1007/s12672-025-02728-4. Discov Oncol. 2025. PMID: 40394417 Free PMC article. Review.
-
Pan-Cancer Analysis of KANK2: Clinical and Molecular Insights into Tumor Progression and Therapeutic Implications.J Cancer. 2025 Jan 6;16(4):1149-1166. doi: 10.7150/jca.105098. eCollection 2025. J Cancer. 2025. PMID: 39895803 Free PMC article.
-
Single-cell RNA sequencing of adult primate neocortex reveals the regulatory dynamics of neural plasticity.Am J Transl Res. 2025 Apr 15;17(4):2562-2576. doi: 10.62347/ZEOR5569. eCollection 2025. Am J Transl Res. 2025. PMID: 40385068 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

