Cold exposure protects against medial arterial calcification development via autophagy
- PMID: 37461031
- PMCID: PMC10351118
- DOI: 10.1186/s12951-023-01985-1
Cold exposure protects against medial arterial calcification development via autophagy
Erratum in
-
Correction: Cold exposure protects against medial arterial calcification development via autophagy.J Nanobiotechnology. 2023 Aug 29;21(1):306. doi: 10.1186/s12951-023-02047-2. J Nanobiotechnology. 2023. PMID: 37644539 Free PMC article. No abstract available.
Abstract
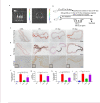
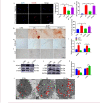
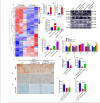
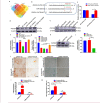
Medial arterial calcification (MAC), a systemic vascular disease different from atherosclerosis, is associated with an increased incidence of cardiovascular events. Several studies have demonstrated that ambient temperature is one of the most important factors affecting cardiovascular events. However, there has been limited research on the effect of different ambient temperatures on MAC. In the present study, we showed that cold temperature exposure (CT) in mice slowed down the formation of vitamin D (VD)-induced vascular calcification compared with room temperature exposure (RT). To investigate the mechanism involved, we isolated plasma-derived exosomes from mice subjected to CT or RT for 30 days (CT-Exo or RT-Exo, respectively). Compared with RT-Exo, CT-Exo remarkably alleviated the calcification/senescence formation of vascular smooth muscle cells (VSMCs) and promoted autophagy by activating the phosphorylation of AMP-activated protein kinase (p-AMPK) and inhibiting phosphorylation of mammalian target of rapamycin (p-mTOR). At the same time, CT-Exo promoted autophagy in β-glycerophosphate (β-GP)-induced VSMCs. The number of autophagosomes and the expression of autophagy-related proteins ATG5 and LC3B increased, while the expression of p62 decreased. Based on a microRNA chip microarray assay and real-time polymerase chain reaction, miR-320a-3p was highly enriched in CT-Exo as well as thoracic aortic vessels in CT mice. miR-320a-3p downregulation in CT-Exo using AntagomiR-320a-3p inhibited autophagy and blunted its anti-calcification protective effect on VSMCs. Moreover, we identified that programmed cell death 4 (PDCD4) is a target of miR-320a-3p, and silencing PDCD4 increased autophagy and decreased calcification in VSMCs. Treatment with CT-Exo alleviated the formation of MAC in VD-treated mice, while these effects were partially reversed by GW4869. Furthermore, the anti-arterial calcification protective effects of CT-Exo were largely abolished by AntagomiR-320a-3p in VD-induced mice. In summary, we have highlighted that prolonged cold may be a good way to reduce the incidence of MAC. Specifically, miR-320a-3p from CT-Exo could protect against the initiation and progression of MAC via the AMPK/mTOR autophagy pathway.
Keywords: Arterial calcification; Autophagy; Cold exposure; PDCD4; Plasma-derived exosomes; Senescence; miR-320a-3p.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Wee NKY, Nguyen AD, Enriquez RF, Zhang L, Herzog H, Baldock PA. Neuropeptide Y Regulation of Energy Partitioning and Bone Mass during Cold exposure. Calcif Tissue Int. 2020;107(5):510–23. - PubMed
-
- Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, Vorwerk D. Multisociety Consensus Quality Improvement revised Consensus Statement for Endovascular Therapy of Acute ischemic stroke. Int J Stroke. 2018;13(6):612–32. - PubMed
-
- Serrat MA. Environmental temperature impact on bone and cartilage growth. Compr Physiol. 2014;4(2):621–55. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

