Trans-scale thermal signaling in biological systems
- PMID: 37461189
- PMCID: PMC10464929
- DOI: 10.1093/jb/mvad053
Trans-scale thermal signaling in biological systems
Abstract
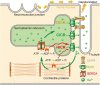
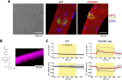
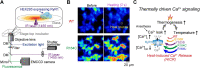
Biochemical reactions in cells serve as the endogenous source of heat, maintaining a constant body temperature. This process requires proper control; otherwise, serious consequences can arise due to the unwanted but unavoidable responses of biological systems to heat. This review aims to present a range of responses to heat in biological systems across various spatial scales. We begin by examining the impaired thermogenesis of malignant hyperthermia in model mice and skeletal muscle cells, demonstrating that the progression of this disease is caused by a positive feedback loop between thermally driven Ca2+ signaling and thermogenesis at the subcellular scale. After we explore thermally driven force generation in both muscle and non-muscle cells, we illustrate how in vitro assays using purified proteins can reveal the heat-responsive properties of proteins and protein assemblies. Building on these experimental findings, we propose the concept of 'trans-scale thermal signaling'.
Keywords: ATPase; fluorescence microscopy; heat-induced calcium release; microheating; type 1 ryanodine receptor. Abbreviations: [Ca2+]i, intracellular Ca2+ concentration; CICR, Ca2+-induced Ca2+ release; ER, endoplasmic reticulum; FDB, flexor digitorum brevis; HEK293 cell, human embryonic kidney 293 cell; HICR, heat-induced Ca2+ release; IP3R, inositol 1,4,5-trisphosphate receptor; MH, malignant hyperthermia; RCC, rapid cooling contracture; RyR1, type 1 ryanodine receptor; SERCA, sarco/endoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum; TRP, transient receptor potential; WT, wild type.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Japanese Biochemical Society.
Figures




References
-
- Nakamura, K., Nakamura, Y., and Kataoka, N. (2022) A hypothalamomedullary network for physiological responses to environmental stresses. Nat. Rev. Neurosci. 23, 35–52 - PubMed
-
- Ikeda, K. and Yamada, T. (2022) Adipose tissue thermogenesis by calcium futile cycling. J. Biochem. 172, 197–203 - PubMed
-
- Matsumura, Y., Osborne, T.F., and Sakai, J. (2022) Epigenetic and environmental regulation of adipocyte function. J. Biochem. 172, 9–16 - PubMed
-
- Schneider, M.F. (1994) Control of calcium-release in functioning skeletal-muscle fibers. Annu. Rev. Physiol. 56, 463–484 - PubMed
-
- Endo, M. (2009) Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 89, 1153–1176 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

