FUS regulates RAN translation through modulating the G-quadruplex structure of GGGGCC repeat RNA in C9orf72-linked ALS/FTD
- PMID: 37461319
- PMCID: PMC10393046
- DOI: 10.7554/eLife.84338
FUS regulates RAN translation through modulating the G-quadruplex structure of GGGGCC repeat RNA in C9orf72-linked ALS/FTD
Abstract
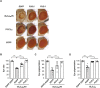
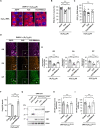
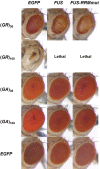
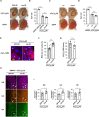
Abnormal expansions of GGGGCC repeat sequence in the noncoding region of the C9orf72 gene is the most common cause of familial amyotrophic lateral sclerosis and frontotemporal dementia (C9-ALS/FTD). The expanded repeat sequence is translated into dipeptide repeat proteins (DPRs) by noncanonical repeat-associated non-AUG (RAN) translation. Since DPRs play central roles in the pathogenesis of C9-ALS/FTD, we here investigate the regulatory mechanisms of RAN translation, focusing on the effects of RNA-binding proteins (RBPs) targeting GGGGCC repeat RNAs. Using C9-ALS/FTD model flies, we demonstrated that the ALS/FTD-linked RBP FUS suppresses RAN translation and neurodegeneration in an RNA-binding activity-dependent manner. Moreover, we found that FUS directly binds to and modulates the G-quadruplex structure of GGGGCC repeat RNA as an RNA chaperone, resulting in the suppression of RAN translation in vitro. These results reveal a previously unrecognized regulatory mechanism of RAN translation by G-quadruplex-targeting RBPs, providing therapeutic insights for C9-ALS/FTD and other repeat expansion diseases.
Keywords: ALS; C9orf72; D. melanogaster; FUS; RAN translation; RNA chaperone; neuroscience; repeat expansion disease.
© 2023, Fujino et al.
Conflict of interest statement
YF, TI, HI, TS, AM, AI, TG, KM, ET, TK, AK, YK, YF, TF, MI, TM, HM, HM, KW, KI, OO, KN, LP, HT No competing interests declared, MU, DO, TT, TT, YN He previously belonged to the Department of Neurotherapeutics, Osaka University Graduate School of Medicine, that is an endowment department supported by Nihon Medi-Physics Co., AbbVie GK., Otsuka Pharm Co., Kyowakai Med. Co., Fujiikai Med. Co., Yukioka Hosp., Osaka Gyoumeikan Hosp., Kyorin Co., and Tokuyukai Med. Co
Figures







Update of
- doi: 10.1101/2022.11.01.514717
- doi: 10.7554/eLife.84338.1
- doi: 10.7554/eLife.84338.2
References
-
- Ash PEA, Bieniek KF, Gendron TF, Caulfield T, Lin W-L, Dejesus-Hernandez M, van Blitterswijk MM, Jansen-West K, Paul JW, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Unconventional translation of C9Orf72 GGGGCC expansion generates insoluble Polypeptides specific to C9Ftd/ALS. Neuron. 2013;77:639–646. doi: 10.1016/j.neuron.2013.02.004. - DOI - PMC - PubMed
-
- Bajc Česnik A, Darovic S, Prpar Mihevc S, Štalekar M, Malnar M, Motaln H, Lee YB, Mazej J, Pohleven J, Grosch M, Modic M, Fonovič M, Turk B, Drukker M, Shaw CE, Rogelj B. Nuclear RNA foci from C9Orf72 expansion Mutation form Paraspeckle-like bodies. Journal of Cell Science. 2019;132:jcs224303. doi: 10.1242/jcs.224303. - DOI - PubMed
-
- Cheng W, Wang S, Mestre AA, Fu C, Makarem A, Xian F, Hayes LR, Lopez-Gonzalez R, Drenner K, Jiang J, Cleveland DW, Sun S. C9Orf72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through Eif2Α Phosphorylation. Nature Communications. 2018;9:51. doi: 10.1038/s41467-017-02495-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

