Engineered hydrogels for mechanobiology
- PMID: 37461429
- PMCID: PMC7614763
- DOI: 10.1038/s43586-022-00179-7
Engineered hydrogels for mechanobiology
Abstract
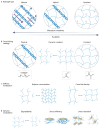
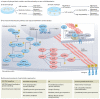
Cells' local mechanical environment can be as important in guiding cellular responses as many well-characterized biochemical cues. Hydrogels that mimic the native extracellular matrix can provide these mechanical cues to encapsulated cells, allowing for the study of their impact on cellular behaviours. Moreover, by harnessing cellular responses to mechanical cues, hydrogels can be used to create tissues in vitro for regenerative medicine applications and for disease modelling. This Primer outlines the importance and challenges of creating hydrogels that mimic the mechanical and biological properties of the native extracellular matrix. The design of hydrogels for mechanobiology studies is discussed, including appropriate choice of cross-linking chemistry and strategies to tailor hydrogel mechanical cues. Techniques for characterizing hydrogels are explained, highlighting methods used to analyze cell behaviour. Example applications for studying fundamental mechanobiological processes and regenerative therapies are provided, along with a discussion of the limitations of hydrogels as mimetics of the native extracellular matrix. The article ends with an outlook for the field, focusing on emerging technologies that will enable new insights into mechanobiology and its role in tissue homeostasis and disease.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures




References
-
- Carey EJ. Direct observations on the transformation of the mesenchyme in the thigh of the pig embryo (Sus scrofa), with especial reference to the genesis of the thigh muscles, of the knee- and hip-joints, and of the primary bone of the femur. J Morphol. 1922;37:1–77.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
