This is a preprint.
The neural basis of defensive behaviour evolution in Peromyscus mice
- PMID: 37461474
- PMCID: PMC10350006
- DOI: 10.1101/2023.07.04.547734
The neural basis of defensive behaviour evolution in Peromyscus mice
Update in
-
The neural basis of species-specific defensive behaviour in Peromyscus mice.Nature. 2025 Sep;645(8080):439-447. doi: 10.1038/s41586-025-09241-2. Epub 2025 Jul 23. Nature. 2025. PMID: 40702175 Free PMC article.
Abstract
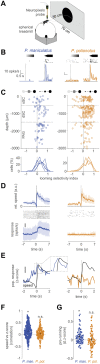
Evading imminent predator threat is critical for survival. Effective defensive strategies can vary, even between closely related species. However, the neural basis of such species-specific behaviours is still poorly understood. Here we find that two sister species of deer mice (genus Peromyscus) show different responses to the same looming stimulus: P. maniculatus, which occupy densely vegetated habitats, predominantly dart to escape, while the open field specialist, P. polionotus, pause their movement. This difference arises from species-specific escape thresholds, is largely context-independent, and can be triggered by both visual and auditory threat stimuli. Using immunohistochemistry and electrophysiological recordings, we find that although visual threat activates the superior colliculus in both species, the role of the dorsal periaqueductal gray (dPAG) in driving behaviour differs. While dPAG activity scales with running speed and involves both excitatory and inhibitory neurons in P. maniculatus, the dPAG is largely silent in P. polionotus, even when darting is triggered. Moreover, optogenetic activation of excitatory dPAG neurons reliably elicits darting behaviour in P. maniculatus but not P. polionotus. Together, we trace the evolution of species-specific escape thresholds to a central circuit node, downstream of peripheral sensory neurons, localizing an ecologically relevant behavioural difference to a specific region of the complex mammalian brain.
Conflict of interest statement
COMPETING INTERESTS The authors declare no competing interests.
Figures




References
REFERENCES (for Main Document)
-
- Abrams P. A., 2000. The evolution of predator-prey interactions: Theory and evidence. Annual Review of Ecology and Systematics 31: 79–105.
-
- Ball W., and Tronick E., 1971. Infant responses to impending collision: Optical and real. Science 171: 818–820. - PubMed
REFERENCES (for Materials and Methods)
-
- Brainard D. H., 1997. The psychophysics toolbox. Spatial Vision 10: 433–436. - PubMed
-
- De Franceschi G., Vivattanasarn T., Saleem Aman B. and Solomon Samuel G., 2016. Vision guides selection of freeze or flight defense strategies in mice. Current Biology 26: 2150–2154. - PubMed
-
- Ho J., Tumkaya T., Aryal S., Choi H. and Claridge-Chang A., 2019. Moving beyond P values: data analysis with estimation graphics. Nature Methods 16: 565–566. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
