This is a preprint.
Molecular recognition of an aversive odorant by the murine trace amine-associated receptor TAAR7f
- PMID: 37461561
- PMCID: PMC10350033
- DOI: 10.1101/2023.07.07.547762
Molecular recognition of an aversive odorant by the murine trace amine-associated receptor TAAR7f
Update in
-
Molecular recognition of an odorant by the murine trace amine-associated receptor TAAR7f.Nat Commun. 2024 Aug 30;15(1):7555. doi: 10.1038/s41467-024-51793-w. Nat Commun. 2024. PMID: 39215004 Free PMC article.
Abstract
There are two main families of G protein-coupled receptors that detect odours in humans, the odorant receptors (ORs) and the trace amine-associated receptors (TAARs). Their amino acid sequences are distinct, with the TAARs being most similar to the aminergic receptors such as those activated by adrenaline, serotonin and histamine. To elucidate the structural determinants of ligand recognition by TAARs, we have determined the cryo-EM structure of a murine receptor, mTAAR7f, coupled to the heterotrimeric G protein Gs and bound to the odorant N,N-dimethylcyclohexylamine (DMCH) to an overall resolution of 2.9 Å. DMCH is bound in a hydrophobic orthosteric binding site primarily through van der Waals interactions and a strong charge-charge interaction between the tertiary amine of the ligand and an aspartic acid residue. This site is distinct and non-overlapping with the binding site for the odorant propionate in the odorant receptor OR51E2. The structure, in combination with mutagenesis data and molecular dynamics simulations suggests that the activation of the receptor follows a similar pathway to that of the β-adrenoceptors, with the significant difference that DMCH interacts directly with one of the main activation microswitch residues.
Figures



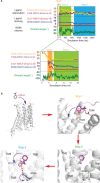

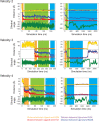




References
-
- Firestein S. How the olfactory system makes sense of scents. Nature 413, 211–218 (2001). - PubMed
-
- Serizawa S. et al. Negative Feedback Regulation Ensures the One Receptor-One Olfactory Neuron Rule in Mouse. Science 302, 2088–2094 (2003). - PubMed
-
- Gainetdinov R. R., Hoener M. C. & Berry M. D. Trace Amines and Their Receptors. Pharmacol. Rev. 70, 549–620 (2018). - PubMed
-
- Li Q. & Liberles S. D. Odor Sensing by Trace Amine-Associated Receptors. in Chemosensory Transduction 67–80 (Elsevier, 2016). doi: 10.1016/B978-0-12-801694-7.00004-4. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
