This is a preprint.
The acid-sensing nociceptor TRPV1 controls breast cancer progression in bone via regulating HGF secretion from sensory neurons
- PMID: 37461623
- PMCID: PMC10350177
- DOI: 10.21203/rs.3.rs-3105966/v1
The acid-sensing nociceptor TRPV1 controls breast cancer progression in bone via regulating HGF secretion from sensory neurons
Abstract
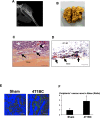
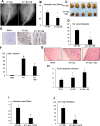
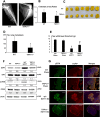
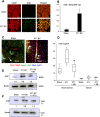
Cancers showing excessive innervation of sensory neurons (SN) in their microenvironments are associated with poor outcomes due to promoted growth, increased tumor recurrence, metastasis, and cancer pain, suggesting SNs play a regulatory role in cancer aggressiveness. Using a preclinical model in which mouse 4T1 breast cancer (BC) cells were injected into the bone marrow of tibiae, we found 4T1 BC cells aggressively colonized bone with bone destruction and subsequently spread to the lung. Of note, 4T1 BC colonization induced the acidic tumor microenvironment in bone in which SNs showed increased innervation and excitation with elevated expression of the acid-sensing nociceptor transient receptor potential vanilloid-1 (TRPV1), eliciting bone pain (BP) assessed by mechanical hypersensitivity. Further, these excited SNs produced increased hepatocyte growth factor (HGF). Importantly, the administration of synthetic and natural TRPV1 antagonists and genetic deletion of TRPV1 decreased HGF production in SNs and inhibited 4T1 BC colonization in bone, pulmonary metastasis from bone, and BP induction. Our results suggest the TRPV1 of SNs promotes BC colonization in bone and lung metastasis via up-regulating HGF production in SNs. The SN TRPV1 may be a novel therapeutic target for BC growing in the acidic bone microenvironment and for BP.
Figures



Similar articles
-
Decreased sensory nerve excitation and bone pain associated with mouse Lewis lung cancer in TRPV1-deficient mice.J Bone Miner Metab. 2018 May;36(3):274-285. doi: 10.1007/s00774-017-0842-7. Epub 2017 May 17. J Bone Miner Metab. 2018. PMID: 28516219
-
The HMGB1/RAGE axis induces bone pain associated with colonization of 4T1 mouse breast cancer in bone.J Bone Oncol. 2020 Oct 28;26:100330. doi: 10.1016/j.jbo.2020.100330. eCollection 2021 Feb. J Bone Oncol. 2020. PMID: 33204606 Free PMC article.
-
Sensory nerves: A driver of the vicious cycle in bone metastasis?J Bone Oncol. 2021 Aug 25;30:100387. doi: 10.1016/j.jbo.2021.100387. eCollection 2021 Oct. J Bone Oncol. 2021. PMID: 34504741 Free PMC article. Review.
-
Crosstalk Between Sensory Nerves and Cancer in Bone.Curr Osteoporos Rep. 2018 Dec;16(6):648-656. doi: 10.1007/s11914-018-0489-x. Curr Osteoporos Rep. 2018. PMID: 30343404 Review.
-
Parathyroid Hormone-Related Peptide Elicits Peripheral TRPV1-dependent Mechanical Hypersensitivity.Front Cell Neurosci. 2018 Feb 15;12:38. doi: 10.3389/fncel.2018.00038. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29497363 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

