This is a preprint.
Double and triple thermodynamic mutant cycles reveal the basis for specific MsbA-lipid interactions
- PMID: 37461710
- PMCID: PMC10350010
- DOI: 10.1101/2023.07.03.547565
Double and triple thermodynamic mutant cycles reveal the basis for specific MsbA-lipid interactions
Update in
- This article has been published with doi: 10.7554/eLife.91094.3
-
Double and triple thermodynamic mutant cycles reveal the basis for specific MsbA-lipid interactions.Elife. 2024 Jan 22;12:RP91094. doi: 10.7554/eLife.91094. Elife. 2024. PMID: 38252560 Free PMC article.
Abstract
Structural and functional studies of the ATP-binding cassette transporter MsbA have revealed two distinct lipopolysaccharide (LPS) binding sites: one located in the central cavity and the other at a membrane-facing, exterior site. Although these binding sites are known to be important for MsbA function, the thermodynamic basis for these specific MsbA-LPS interactions is not well understood. Here, we use native mass spectrometry to determine the thermodynamics of MsbA interacting with the LPS-precursor 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo)2-lipid A (KDL). The binding of KDL is solely driven by entropy, despite the transporter adopting an inward-facing conformation or trapped in an outward-facing conformation with adenosine 5'-diphosphate and vanadate. An extension of the mutant cycle approach is employed to probe basic residues that interact with KDL. We find the molecular recognition of KDL is driven by a positive coupling entropy (as large as -100 kJ/mol at 298K) that outweighs unfavorable coupling enthalpy. These findings indicate that alterations in solvent reorganization and conformational entropy can contribute significantly to the free energy of protein-lipid association. The results presented herein showcase the advantage of native MS to obtain thermodynamic insight into protein-lipid interactions that would otherwise be intractable using traditional approaches, and this enabling technology will be instrumental in the life sciences and drug discovery.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures



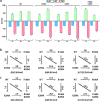
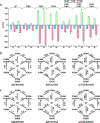

Similar articles
-
Double and triple thermodynamic mutant cycles reveal the basis for specific MsbA-lipid interactions.Elife. 2024 Jan 22;12:RP91094. doi: 10.7554/eLife.91094. Elife. 2024. PMID: 38252560 Free PMC article.
-
Structural basis for lipid and copper regulation of the ABC transporter MsbA.Nat Commun. 2022 Nov 26;13(1):7291. doi: 10.1038/s41467-022-34905-2. Nat Commun. 2022. PMID: 36435815 Free PMC article.
-
Native mass spectrometry and structural studies reveal modulation of MsbA-nucleotide interactions by lipids.Nat Commun. 2024 Jul 15;15(1):5946. doi: 10.1038/s41467-024-50350-9. Nat Commun. 2024. PMID: 39009687 Free PMC article.
-
The sugar 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) as a characteristic component of bacterial endotoxin -- a review of its biosynthesis, function, and placement in the lipopolysaccharide core.Can J Microbiol. 2013 Oct;59(10):645-55. doi: 10.1139/cjm-2013-0490. Epub 2013 Oct 1. Can J Microbiol. 2013. PMID: 24102217 Review.
-
MsbA: an ABC transporter paradigm.Biochem Soc Trans. 2021 Dec 17;49(6):2917-2927. doi: 10.1042/BST20211030. Biochem Soc Trans. 2021. PMID: 34821931 Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
