Chylomicrons Regulate Lacteal Permeability and Intestinal Lipid Absorption
- PMID: 37462027
- PMCID: PMC10530007
- DOI: 10.1161/CIRCRESAHA.123.322607
Chylomicrons Regulate Lacteal Permeability and Intestinal Lipid Absorption
Abstract
Background: Lymphatic vessels are responsible for tissue drainage, and their malfunction is associated with chronic diseases. Lymph uptake occurs via specialized open cell-cell junctions between capillary lymphatic endothelial cells (LECs), whereas closed junctions in collecting LECs prevent lymph leakage. LEC junctions are known to dynamically remodel in development and disease, but how lymphatic permeability is regulated remains poorly understood.
Methods: We used various genetically engineered mouse models in combination with cellular, biochemical, and molecular biology approaches to elucidate the signaling pathways regulating junction morphology and function in lymphatic capillaries.
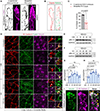
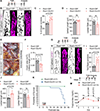
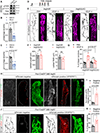
Results: By studying the permeability of intestinal lacteal capillaries to lipoprotein particles known as chylomicrons, we show that ROCK (Rho-associated kinase)-dependent cytoskeletal contractility is a fundamental mechanism of LEC permeability regulation. We show that chylomicron-derived lipids trigger neonatal lacteal junction opening via ROCK-dependent contraction of junction-anchored stress fibers. LEC-specific ROCK deletion abolished junction opening and plasma lipid uptake. Chylomicrons additionally inhibited VEGF (vascular endothelial growth factor)-A signaling. We show that VEGF-A antagonizes LEC junction opening via VEGFR (VEGF receptor) 2 and VEGFR3-dependent PI3K (phosphatidylinositol 3-kinase)/AKT (protein kinase B) activation of the small GTPase RAC1 (Rac family small GTPase 1), thereby restricting RhoA (Ras homolog family member A)/ROCK-mediated cytoskeleton contraction.
Conclusions: Our results reveal that antagonistic inputs into ROCK-dependent cytoskeleton contractions regulate the interconversion of lymphatic junctions in the intestine and in other tissues, providing a tunable mechanism to control the lymphatic barrier.
Keywords: chylomicrons; endothelial cells; lipid; permeability; vascular endothelial growth factor A.
Conflict of interest statement
Figures


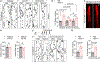

Comment in
-
Mechanisms of Lymphatic Endothelial Cell Junction Transformations.Circ Res. 2023 Aug 4;133(4):350-352. doi: 10.1161/CIRCRESAHA.123.323210. Epub 2023 Aug 3. Circ Res. 2023. PMID: 37535754 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

