An expanded safety/feasibility study of the EMulate Therapeutics Voyager™ System in patients with recurrent glioblastoma
- PMID: 37462385
- PMCID: PMC10410686
- DOI: 10.2217/cns-2022-0016
An expanded safety/feasibility study of the EMulate Therapeutics Voyager™ System in patients with recurrent glioblastoma
Abstract
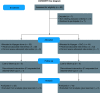
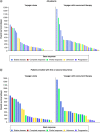
Aim: The EMulate Therapeutics Voyager™ is a simple, wearable, home-use device that uses an alternating electromagnetic field to alter biologic signaling within cells. Objective: To assess the safety/feasibility of the Voyager in the treatment of recurrent glioblastoma (rGBM). Methods: In this study, patients with rGBM were treated with Voyager as monotherapy or in combination with standard chemotherapy at the Investigator's discretion. Safety was assessed by incidence of adverse events associated with the Voyager. Patients were followed until death. Results: A total of 75 patients were enrolled and treated for at least one day with the Voyager (safety population). Device-related adverse events were uncommon and generally did not result in interruption or withdrawal from treatment. There were no serious adverse events associated with Voyager. A total of 60 patients were treated for at least one month (clinical utility population). The median progression-free survival (PFS) was 17 weeks (4.3 months) in the Voyager only group (n = 24) and 21 weeks (5.3 months) in the Voyager + concurrent therapy group (n = 36). The median overall survival (OS) was 7 months in the Voyager only group and 9 months in the Voyager + concurrent therapy group. In patients treated with Voyager + concurrent therapy, the median OS for patients enrolled with their 1st or 2nd recurrence (n = 26) was 10 months, while in patients enrolled with their 3rd or 4th recurrence (n = 10) OS was 7 months. Conclusion: The data support the safety and feasibility of the Voyager for the treatment of rGBM. Further prospective study of the device is warranted. Trial Registration Number: NCT02296580 (ClinicalTrials.gov).
Keywords: GBM; brain tumor; clinical trial; electromagnetic field; medical device; novel therapy; recurrent glioblastoma.
Conflict of interest statement
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures
References
-
- Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 131(6), 803–820 (2016). - PubMed
-
- Stupp R, Taillibert S, Kanner A et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 318(23), 2306–2316 (2017). - PMC - PubMed
-
•• First paper showing positive effects of electromagnetic fields in brain tumors.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
