Impact of Complete Revascularization in the ISCHEMIA Trial
- PMID: 37462593
- PMCID: PMC10529674
- DOI: 10.1016/j.jacc.2023.06.015
Impact of Complete Revascularization in the ISCHEMIA Trial
Abstract
Background: Anatomic complete revascularization (ACR) and functional complete revascularization (FCR) have been associated with reduced death and myocardial infarction (MI) in some prior studies. The impact of complete revascularization (CR) in patients undergoing an invasive (INV) compared with a conservative (CON) management strategy has not been reported.
Objectives: Among patients with chronic coronary disease without prior coronary artery bypass grafting randomized to INV vs CON management in the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial, we examined the following: 1) the outcomes of ACR and FCR compared with incomplete revascularization; and 2) the potential impact of achieving CR in all INV patients compared with CON management.
Methods: ACR and FCR in the INV group were assessed at an independent core laboratory. Multivariable-adjusted outcomes of CR were examined in INV patients. Inverse probability weighted modeling was then performed to estimate the treatment effect had CR been achieved in all INV patients compared with CON management.
Results: ACR and FCR were achieved in 43.4% and 58.4% of 1,824 INV patients. ACR was associated with reduced 4-year rates of cardiovascular death or MI compared with incomplete revascularization. By inverse probability weighted modeling, ACR in all 2,296 INV patients compared with 2,498 CON patients was associated with a lower 4-year rate of cardiovascular death or MI (difference -3.5; 95% CI: -7.2% to 0.0%). In comparison, the event rate difference of cardiovascular death or MI for INV minus CON in the overall ISCHEMIA trial was -2.4%. Results were similar but less pronounced with FCR.
Conclusions: The outcomes of an INV strategy may be improved if CR (especially ACR) is achieved. (International Study of Comparative Health Effectiveness With Medical and Invasive Approaches [ISCHEMIA]; NCT01471522).
Keywords: complete revascularization; coronary artery disease; ischemia; prognosis; revascularization.
Copyright © 2023 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by National Institutes of Health grants U01HL105907, U01HL105462, U01HL105561, U01HL105565, T32 HL079896. This project was also supported in part by Clinical Translational Science Award No. 11UL1 TR001445 from the National Center for Advancing Translational Sciences and by grants from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Devices or medications were provided by Abbott Vascular (previously St Jude Medical, Inc), Medtronic, Inc, Phillips (previously Volcano Corporation), and Omron Healthcare, Inc; and medications were provided by Amgen Inc, Arbor Pharmaceuticals, LLC, AstraZeneca Pharmaceuticals, LP, Espero Pharmaceuticals, Merck Sharp & Dohme Corp, and Sunovion Pharmaceuticals. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences, the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the Department of Health and Human Services. Dr Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, and Abbott; has served as a consultant to Valfix, TherOx, Robocath, HeartFlow, Ablative Solutions, Vectorious, Miracor, Neovasc, Ancora, Elucid Bio, Occlutech, CorFlow, Apollo Therapeutics, Impulse Dynamics, Cardiomech, Gore, Amgen, Adona Medical, and Millennia Biopharma; has equity/options from Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, Valfix, and Xenter; his daughter is an employee at IQVIA; and his employer, Mount Sinai Hospital, receives research support from Abbott, Abiomed, Bioventrix, Cardiovascular Systems Inc, Phillips, Biosense Webster, Shockwave, Vascular Dynamics, Pulnovo, and V-wave. Dr Ali has received institutional grant support from Abbott, Abiomed, Acist Medical, Boston Scientific, Cardiovascular Systems Inc, Medtronic Inc, National Institute of Health, Opsens Medical, Philips, and Teleflex; has received consulting fees from AstraZeneca, Philips, and Shockwave; and has equity in Elucid, Spectrawave, Shockwave, and VitalConnect. Dr Genereux has received consulting, advisory, and speaker fees from Abbott Vascular, Abiomed, Edwards Lifesciences, Medtronic, and BioTrace Medical; has served as a consultant for Boston Scientific, CARANX Medica, Cardiovascular System Inc (PI Eclipse Trial), GE Healthcare, iRythm Technologies, Opsens, Siemens, Teleflex, and 4C Medical (PI Feasibility study); has equity in and served as a consultant for Pi-Cardia, Puzzle Medical, Saranas, and Soundbite Medical Inc; has served as a consultant for and received speaker fees from Shockwave; and has served as a proctor for, received an institutional research grant from, and served as PI for the EARLY-TAVR and PROGRESS trials for Edwards Lifesciences. Dr Bangalore has received grants from the National Heart, Lung, and Blood Institute; has received grants and personal fees from Abbott Vascular; and has received personal fees from Biotronik, Pfizer, Amgen, and Reata. Dr Mavromatis has received grants from the National Heart, Lung, and Blood Institute, National Heart, Lung, and Blood Institute (CV Inflammation Reduction Trial and GMCSF in PAD-3 Trial), CSL Behring, St Jude Medical, Medtronic, DalCor Pharmaceuticals, AstraZeneca, Novartis, and Regeneron; and is a Member of the American College of Cardiology and Society of Cardiovascular Angiography and Interventions. Dr Dwivedi has received grants from the National Heart, Lung, and Blood Institute. Dr Chen has received grants from the National Heart, Lung, and Blood Institute. Dr Chaitman has received grants from the National Heart, Lung, and Blood Institute; and has received personal fees from Merck, Novo Nordisk, Sanofi, Lilly, Johnson and Johnson, Daiichi-Sankyo, Tricida, Relypsa, Imbria, and Xylocor outside the submitted work. Dr Berman has received royalties for nuclear cardiology software from Cedars-Sinai Medical Center. Dr Boden has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received grants from Abbvie, Amarin, and Amgen; and has received personal fees from Amgen, Cleveland Clinic Clinical Coordinating Center, and Janssen. Dr White has received grant support paid to the institution and fees for serving on Steering Committees from Sanofi, Regeneron Pharmaceuticals, Eli Lilly, Omthera Pharmaceuticals, American Regent, Eisai Inc, DalCor Pharma UK, Inc, CSL Behring, NHI, Sanofi Australia Pty Ltd, Esperion Therapeutics, Inc, and the National Institutes of Health; and has served on the Advisory Board for Genentech, Inc (an affiliate of F. Hoffmann-La Roche Ltd, ‘Roche’; Lytics Post-PCI Advisory Board at European Society of Cardiology). Dr Fremes has received NPI CIHR funding for the ROMA and STICH3C studies. Dr Reynolds has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; and has received nonfinancial support from Abbott Vascular, Siemens, and BioTelemetry. Dr Spertus has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study; has received personal fees from Bayer, Novartis, AstraZeneca, Amgen, Janssen, and United Healthcare; has received grants from American College of Cardiology; has received personal fees from Blue Cross Blue Shield of Kansas City; and has a patent Copyright to Seattle Angina Questionnaire, with royalties paid and Equity in Health Outcomes Sciences. Dr Hochman is PI for the ISCHEMIA trial for which, in addition to support by the National Heart, Lung, and Blood Institute grant, devices and medications were provided by Medtronic, Inc, Abbott Vascular, Inc (formerly St Jude Medical, Inc), Royal Philips NV (formerly Volcano Corporation), Arbor Pharmaceuticals, LLC, AstraZeneca Pharmaceuticals, LP, Merck Sharp & Dohme Corp, Omron Healthcare, Inc, Sunovion Pharmaceuticals, Inc, Espero BioPharma, and Amgen Inc; and has received financial donations from Arbor Pharmaceuticals LLC and AstraZeneca Pharmaceuticals LP. Dr Maron has received grants from the National Heart, Lung, and Blood Institute during the conduct of the study. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
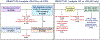
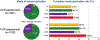

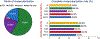


Comment in
-
Complete Coronary Revascularization: A New Strategy to Improve Clinical Outcomes for Stable Coronary Artery Disease?J Am Coll Cardiol. 2023 Sep 19;82(12):1189-1191. doi: 10.1016/j.jacc.2023.07.008. J Am Coll Cardiol. 2023. PMID: 37704308 No abstract available.
-
Drawing Conclusions From Exploratory Analyses: A Slippery Slope.J Am Coll Cardiol. 2024 Jan 30;83(4):e31. doi: 10.1016/j.jacc.2023.10.050. J Am Coll Cardiol. 2024. PMID: 38267116 No abstract available.
-
And Yet it Moves: E Pur Si Muove.J Am Coll Cardiol. 2024 Jan 30;83(4):e33. doi: 10.1016/j.jacc.2023.10.049. J Am Coll Cardiol. 2024. PMID: 38267117 No abstract available.
-
Functional vs Anatomical Complete Revascularization: Is There an "ISCHEMIA" of FFR?J Am Coll Cardiol. 2024 Jan 30;83(4):e35. doi: 10.1016/j.jacc.2023.10.048. J Am Coll Cardiol. 2024. PMID: 38267118 No abstract available.
-
Benefit of Complete Anatomic Revascularization in ISCHEMIA: Reprise of a Familiar Theme.J Am Coll Cardiol. 2024 Jan 30;83(4):e37. doi: 10.1016/j.jacc.2023.10.047. J Am Coll Cardiol. 2024. PMID: 38267119 No abstract available.
References
-
- Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. - PubMed
-
- Mehta SR, Wood DA, Storey RF, et al. Complete Revascularization with Multivessel PCI for Myocardial Infarction. N Engl J Med. 2019;381:1411–1421. - PubMed
-
- Gaba P, Gersh BJ, Ali ZA, Moses JW, Stone GW. Complete versus incomplete coronary revascularization: definitions, assessment and outcomes. Nat Rev Cardiol. 2021;18:155–168. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

