Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial
- PMID: 37462600
- PMCID: PMC10528593
- DOI: 10.1016/j.jaci.2023.05.024
Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial
Abstract
Background: Eosinophilic gastritis/gastroenteritis (EoG/EoGE) are rare disorders with pathologic gastric and/or small intestinal eosinophilia lacking an approved therapy. An allergic mechanism is postulated but underexplored mechanistically and therapeutically.
Objective: We evaluated the effectiveness of a food allergen-free diet (elemental formula) in controlling gastrointestinal eosinophilia in adult EoG/EoGE.
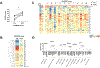
Methods: Adults aged 18 to 65 years with histologically active EoG/EoGE (≥30 eosinophils per high-power field) in the stomach and/or duodenum and gastrointestinal symptoms within the month preceding enrollment were prospectively enrolled onto a single-arm clinical trial to receive elemental formula for 6 consecutive weeks. The primary end point was percentage of participants with complete histologic remission (<30 eosinophils per high-power field in both stomach and duodenum). Exploratory outcomes were improvement in symptoms, endoscopy results, blood eosinophilia, quality of life, Physician Global Assessment score, and EoG-relevant gastric transcriptome and microbiome.
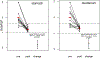
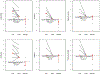
Results: Fifteen adults (47% male, average age 37.7 years, average symptom duration 8.8 years) completed the trial. Multi-gastrointestinal segment involvement affected 87%. All subjects had complete histologic remission in the stomach (P = .002) and duodenum (P = .001). Scores improved in overall PhGA (P = .002); EGREFS (P = .003); EGDP (P = .002); SODA pain intensity (P = .044), non-pain (P = .039), and satisfaction (P = .0024); and PROMIS depression (P = .0078) and fatigue (P = .04). Food reintroduction reversed these improvements. The intervention was well tolerated in 14 subjects, with 1 serious adverse event reported in 1 subject.
Conclusion: An amino acid-based elemental diet improves histologic, endoscopic, symptomatic, quality-of-life, and molecular parameters of EoG/EoGE; these findings and disease recurrence with food trigger reintroduction support a dominant role for food allergens in disease pathogenesis.
Clinicaltrials: gov Identifier: NCT03320369.
Keywords: Eosinophilic gastritis; elemental diet; eosinophilia; eosinophilic gastroenteritis; food allergy.
Copyright © 2023 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest
Nirmala Gonsalves: N.G. serves as a consultant (Allakos, Sanofi-Regeneron, AstraZeneca, Abbvie, Nutricia, Knopp Pharma, BMS) and as part of the speakers bureau (Takeda) (Sanofi-Regeneron) and receives royalties (Up-to-Date).
Bethany Doerfler: BD serves as consultant for Trellus LLC and on speakers bureau for Nutricia
Angelika Zalewski: None
Guang-Yu Yang: None
Lisa Martin: None
Xue Zhang: None
Tetsuo Shoda: T.S. is a co-inventor of patents for the EG Diagnostic Panel owned by Cincinnati Children’s Hospital Medical Center.
Michael Brusilovsky: None
Seema Aceves: S.S. Aceves declares being a consultant for AstraZeneca and Regeneron, a speaker for MedScape and Sanofi-Genzyme (Regeneron), and a coinventor of oral viscous budesonide (patented by the University of California, San Diego and licensed by Takeda).
Kathy Thompson: K.T.’s co-authorship of this publication does not necessarily constitute endorsement by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or any other agency of the United States government
Amanda K. Rudman Spergel: A.K.R.S.’s co-authorship of this publication does not necessarily constitute endorsement by the National Institute of Allergy and Infectious Diseases, the National Institutes of Health or any other agency of the United States government
Glenn Furuta: G.F. is a founder of EnteroTrack and a consultant for Shire/Takeda.
Marc Rothenberg: M.E.R. is a consultant for Pulm One, Spoon Guru, ClostraBio, Serpin Pharm, Allakos, Celldex, Bristol Myers Squibb, AstraZeneca, Ellodi Pharma, GlaxoSmithKline, Regeneron/Sanofi, Revolo Biotherapeutics, and Guidepoint and has an equity interest in the first six listed and royalties from reslizumab (Teva Pharmaceuticals), PEESSv2 (Mapi Research Trust), and UpToDate. M.E.R. is an inventor of patents owned by Cincinnati Children’s Hospital.
Ikuo Hirano: I.H. receives research funding (Adare/Ellodi, Allakos, Arena, AstraZeneca, Meritage, Celgene/Receptos/BMS, Regeneron, Shire/Takeda) and is a consultant (Adare/Ellodi, Allakos, Amgen, Arena, AstraZeneca, Celgene/Receptos/BMS, Eli Lilly, EsoCap, Gossamer Bio, Parexel/Calyx, Phathom, Regeneron/Sanofi, Shire/Takeda).
Figures
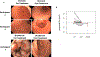
References
-
- Chang JY, Choung RS, Lee RM, Locke GR, Schleck CD, Zinsmeister AR, et al. A shift in the clinical spectrum of eosinophilic gastroenteritis toward the mucosal disease type. Clin Gastroenterol Hepatol. 2010;8(8):669–75; quiz e88. - PubMed
-
- Pineton de Chambrun G, Gonzalez F, Canva JY, Gonzalez S, Houssin L, Desreumaux P, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950–6.e1. - PubMed
-
- Collins MH. Histopathologic features of eosinophilic esophagitis and eosinophilic gastrointestinal diseases. Gastroenterol Clin North Am. 2014;43(2):257–68. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

