Development of an Affordable ELISA Targeting the SARS-CoV-2 Nucleocapsid and Its Application to Samples from the Ongoing COVID-19 Epidemic in Ghana
- PMID: 37462793
- PMCID: PMC10435612
- DOI: 10.1007/s40291-023-00655-0
Development of an Affordable ELISA Targeting the SARS-CoV-2 Nucleocapsid and Its Application to Samples from the Ongoing COVID-19 Epidemic in Ghana
Abstract
Introduction: The true nature of the population spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in populations is often not fully known as most cases, particularly in Africa, are asymptomatic. Finding the true magnitude of SARS-CoV-2 spread is crucial to provide actionable data about the epidemiological progress of the disease for researchers and policymakers. This study developed and optimized an antibody enzyme-linked immunosorbent assay (ELISA) using recombinant nucleocapsid antigen expressed in-house using a simple bacterial expression system.
Methods: Nucleocapsid protein from SARS-CoV-2 was expressed and purified from Escherichia coli. Plasma samples used for the assay development were obtained from Ghanaian SARS-CoV-2 seropositive individuals during the pandemic, while seronegative controls were plasma samples collected from blood donors before the coronavirus disease 2019 (COVID-19) pandemic. Another set of seronegative controls was collected during the COVID-19 pandemic. Antibody detection and levels within the samples were validated using commercial kits and Luminex. Analyses were performed using GraphPad Prism, and the sensitivity, specificity and background cut-off were calculated.
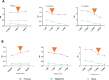
Results and discussion: This low-cost ELISA (£0.96/test) assay has a high prediction of 98.9%, and sensitivity and specificity of 97% and 99%, respectively. The assay was subsequently used to screen plasma from SARS-CoV-2 RT-PCR-positive Ghanaians. The assay showed no significant difference in nucleocapsid antibody levels between symptomatic and asymptomatic, with an increase of the levels over time. This is in line with our previous publication.
Conclusion: This study developed a low-cost and transferable assay that enables highly sensitive and specific detection of human anti-SARS-CoV-2 IgG antibodies. This assay can be modified to include additional antigens and used for continuous monitoring of sero-exposure to SARS-CoV-2 in West Africa.
© 2023. The Author(s).
Conflict of interest statement
Authors; KT, PCO, FYN, BT, GKS, MVH, ST, AIB, KA, SKA, AAM, SNAA, LO, DQ, SL, CA, DD, EKO, DO-M, WA, ET, OQ, IOD, JA, YA, MC, JM, JCR, GAA, YB, CJM and PKQ declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures



References
-
- WHO. Tracking SARS-CoV-2 variants. World Health Organisation. 2022;:1–20. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 23 Nov 2022.
-
- Quashie PK, Mutungi JK, Dzabeng F, Oduro-Mensah D, Opurum PC, Tapela K, et al. Trends of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody prevalence in selected regions across Ghana. Wellcome Open Res. 2021;6:173. doi: 10.12688/wellcomeopenres.16890.1. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

