TLR7/8 stress response drives histiocytosis in SLC29A3 disorders
- PMID: 37462944
- PMCID: PMC10354536
- DOI: 10.1084/jem.20230054
TLR7/8 stress response drives histiocytosis in SLC29A3 disorders
Abstract
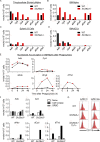
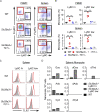
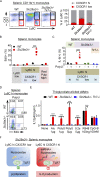
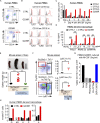
Loss-of-function mutations in the lysosomal nucleoside transporter SLC29A3 cause lysosomal nucleoside storage and histiocytosis: phagocyte accumulation in multiple organs. However, little is known about the mechanism by which lysosomal nucleoside storage drives histiocytosis. Herein, histiocytosis in Slc29a3-/- mice was shown to depend on Toll-like receptor 7 (TLR7), which senses a combination of nucleosides and oligoribonucleotides (ORNs). TLR7 increased phagocyte numbers by driving the proliferation of Ly6Chi immature monocytes and their maturation into Ly6Clow phagocytes in Slc29a3-/- mice. Downstream of TLR7, FcRγ and DAP10 were required for monocyte proliferation. Histiocytosis is accompanied by inflammation in SLC29A3 disorders. However, TLR7 in nucleoside-laden splenic monocytes failed to activate inflammatory responses. Enhanced production of proinflammatory cytokines was observed only after stimulation with ssRNAs, which would increase lysosomal ORNs. Patient-derived monocytes harboring the G208R SLC29A3 mutation showed enhanced survival and proliferation in a TLR8-antagonist-sensitive manner. These results demonstrated that TLR7/8 responses to lysosomal nucleoside stress drive SLC29A3 disorders.
© 2023 Shibata et al.
Conflict of interest statement
Disclosures: T. Shibata reported non-financial support from Invivogen outside the submitted work. Additionly, T. Shibata has a patent to Human TLR8 Tg pending. K. Miyake reported grants from Daiichi Sankyo Co. Ltd. outside the submitted work. Additionly, K. Miyake had a patent to P2018-193376A issued by The University of Tokyo. No other disclosures were reported.
Figures







References
-
- Aluri, J., Bach A., Kaviany S., Chiquetto Paracatu L., Kitcharoensakkul M., Walkiewicz M.A., Putnam C.D., Shinawi M., Saucier N., Rizzi E.M., et al. . 2021. Immunodeficiency and bone marrow failure with mosaic and germline TLR8 gain of function. Blood. 137:2450–2462. 10.1182/blood.2020009620 - DOI - PMC - PubMed
-
- Baldwin, S.A., Yao S.Y.M., Hyde R.J., Ng A.M.L., Foppolo S., Barnes K., Ritzel M.W.L., Cass C.E., and Young J.D.. 2005. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J. Biol. Chem. 280:15880–15887. 10.1074/jbc.M414337200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

