The role of Plasmodium V-ATPase in vacuolar physiology and antimalarial drug uptake
- PMID: 37463201
- PMCID: PMC10372686
- DOI: 10.1073/pnas.2306420120
The role of Plasmodium V-ATPase in vacuolar physiology and antimalarial drug uptake
Abstract
To ensure their survival in the human bloodstream, malaria parasites degrade up to 80% of the host erythrocyte hemoglobin in an acidified digestive vacuole. Here, we combine conditional reverse genetics and quantitative imaging approaches to demonstrate that the human malaria pathogen Plasmodium falciparum employs a heteromultimeric V-ATPase complex to acidify the digestive vacuole matrix, which is essential for intravacuolar hemoglobin release, heme detoxification, and parasite survival. We reveal an additional function of the membrane-embedded V-ATPase subunits in regulating morphogenesis of the digestive vacuole independent of proton translocation. We further show that intravacuolar accumulation of antimalarial chemotherapeutics is surprisingly resilient to severe deacidification of the vacuole and that modulation of V-ATPase activity does not affect parasite sensitivity toward these drugs.
Keywords: Plasmodium falciparum; V-ATPase; chloroquine; malaria; vacuole.
Conflict of interest statement
The authors declare no competing interest.
Figures

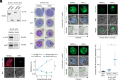

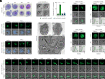

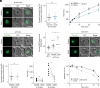
Comment in
-
Inducing indigestion in malaria parasites: Genetic manipulations of a proton pump.Proc Natl Acad Sci U S A. 2023 Aug 22;120(34):e2310870120. doi: 10.1073/pnas.2310870120. Epub 2023 Aug 9. Proc Natl Acad Sci U S A. 2023. PMID: 37556486 Free PMC article. No abstract available.
References
-
- Matz J. M., Beck J. R., Blackman M. J., The parasitophorous vacuole of the blood-stage malaria parasite. Nat. Rev. Microbiol. 18, 379–391 (2020). - PubMed
-
- Spielmann T., Gras S., Sabitzki R., Meissner M., Endocytosis in Plasmodium and Toxoplasma parasites. Trends Parasitol. 36, 520–532 (2020). - PubMed
-
- Matz J. M., Plasmodium’s bottomless pit: Properties and functions of the malaria parasite’s digestive vacuole. Trends Parasitol. 38, 525–543 (2022). - PubMed
-
- Lew V. L., Macdonald L., Ginsburg H., Krugliak M., Tiffert T., Excess haemoglobin digestion by malaria parasites: A strategy to prevent premature host cell lysis. Blood Cells Mol. Dis. 32, 353–359 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

