MicroRNA-335-5p suppresses voltage-gated sodium channel expression and may be a target for seizure control
- PMID: 37463203
- PMCID: PMC10372546
- DOI: 10.1073/pnas.2216658120
MicroRNA-335-5p suppresses voltage-gated sodium channel expression and may be a target for seizure control
Abstract
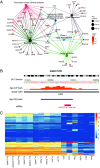
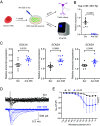
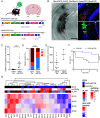
There remains an urgent need for new therapies for treatment-resistant epilepsy. Sodium channel blockers are effective for seizure control in common forms of epilepsy, but loss of sodium channel function underlies some genetic forms of epilepsy. Approaches that provide bidirectional control of sodium channel expression are needed. MicroRNAs (miRNA) are small noncoding RNAs which negatively regulate gene expression. Here we show that genome-wide miRNA screening of hippocampal tissue from a rat epilepsy model, mice treated with the antiseizure medicine cannabidiol, and plasma from patients with treatment-resistant epilepsy, converge on a single target-miR-335-5p. Pathway analysis on predicted and validated miR-335-5p targets identified multiple voltage-gated sodium channels (VGSCs). Intracerebroventricular injection of antisense oligonucleotides against miR-335-5p resulted in upregulation of Scn1a, Scn2a, and Scn3a in the mouse brain and an increased action potential rising phase and greater excitability of hippocampal pyramidal neurons in brain slice recordings, consistent with VGSCs as functional targets of miR-335-5p. Blocking miR-335-5p also increased voltage-gated sodium currents and SCN1A, SCN2A, and SCN3A expression in human induced pluripotent stem cell-derived neurons. Inhibition of miR-335-5p increased susceptibility to tonic-clonic seizures in the pentylenetetrazol seizure model, whereas adeno-associated virus 9-mediated overexpression of miR-335-5p reduced seizure severity and improved survival. These studies suggest modulation of miR-335-5p may be a means to regulate VGSCs and affect neuronal excitability and seizures. Changes to miR-335-5p may reflect compensatory mechanisms to control excitability and could provide biomarker or therapeutic strategies for different types of treatment-resistant epilepsy.
Keywords: adeno-associated virus; antisense oligonucleotides; drug resistance; epilepsy; noncoding RNA.
Conflict of interest statement
RCSI University of Medicine and Health Sciences (D.C.H., G.M., and M.H.) reports the European Patent Application No. EP21198390.3 “Modulation of microRNA-335-5p for the treatment of sodium channelopathies.”
Figures



Comment in
-
MicroRNA 335-5p: The Sodium Channel Silencer.Epilepsy Curr. 2023 Nov 22;24(1):50-52. doi: 10.1177/15357597231212373. eCollection 2024 Jan-Feb. Epilepsy Curr. 2023. PMID: 38327537 Free PMC article.
References
-
- Fisher R. S., et al. , ILAE official report: A practical clinical definition of epilepsy. Epilepsia 55, 475–482 (2014). - PubMed
-
- Brodie M. J., Sodium Channel Blockers in the Treatment of Epilepsy. CNS Drugs 31, 527–534 (2017). - PubMed
-
- Steel D., Symonds J. D., Zuberi S. M., Brunklaus A., Dravet syndrome and its mimics: Beyond SCN1A. Epilepsia 58, 1807–1816 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

