Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks
- PMID: 37463581
- PMCID: PMC10433941
- DOI: 10.1016/j.immuni.2023.06.020
Lymph node medulla regulates the spatiotemporal unfolding of resident dendritic cell networks
Abstract
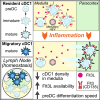
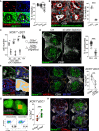
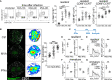
Unlike macrophage networks composed of long-lived tissue-resident cells within specific niches, conventional dendritic cells (cDCs) that generate a 3D network in lymph nodes (LNs) are short lived and continuously replaced by DC precursors (preDCs) from the bone marrow (BM). Here, we examined whether specific anatomical niches exist within which preDCs differentiate toward immature cDCs. In situ photoconversion and Prtn3-based fate-tracking revealed that the LN medullary cords are preferential entry sites for preDCs, serving as specific differentiation niches. Repopulation and fate-tracking approaches demonstrated that the cDC1 network unfolded from the medulla along the vascular tree toward the paracortex. During inflammation, collective maturation and migration of resident cDC1s to the paracortex created discontinuity in the medullary cDC1 network and temporarily impaired responsiveness. The decrease in local cDC1 density resulted in higher Flt3L availability in the medullary niche, which accelerated cDC1 development to restore the network. Thus, the spatiotemporal development of the cDC1 network is locally regulated in dedicated LN niches via sensing of cDC1 densities.
Keywords: CD135; Flt3L; Prtn3; cDC1; conveyor belt; feedback; infection; inflammation; migration; preDC.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
Resident dendritic cell density in the lymph node paracortex is preDC-estined.Immunity. 2023 Aug 8;56(8):1699-1701. doi: 10.1016/j.immuni.2023.07.013. Immunity. 2023. PMID: 37557075
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

