Phosphoinositides in New Spaces
- PMID: 37463718
- PMCID: PMC10513166
- DOI: 10.1101/cshperspect.a041406
Phosphoinositides in New Spaces
Abstract
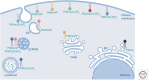
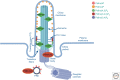
Phosphoinositides (PIs) are phospholipids derived from phosphatidylinositol. PIs are regulated via reversible phosphorylation, which is directed by the opposing actions of PI kinases and phosphatases. PIs constitute a minor fraction of the total cellular lipid pool but play pleiotropic roles in multiple aspects of cell biology. Genetic mutations of PI regulatory enzymes have been identified in rare congenital developmental syndromes, including ciliopathies, and in numerous human diseases, such as cancer and metabolic and neurological disorders. Accordingly, PI regulatory enzymes have been targeted in the design of potential therapeutic interventions for human diseases. Recent advances place PIs as central regulators of membrane dynamics within functionally distinct subcellular compartments. This brief review focuses on the emerging role PIs play in regulating cell signaling within the primary cilium and in directing transfer of molecules at interorganelle membrane contact sites and identifies new roles for PIs in subcellular spaces.
Copyright © 2023 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Azzedine H, Bolino A, Taïeb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, et al. 2003. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot–Marie–Tooth disease associated with early-onset glaucoma. Am J Hum Genet 72: 1141–1153. 10.1086/375034 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
