HCK induces macrophage activation to promote renal inflammation and fibrosis via suppression of autophagy
- PMID: 37463911
- PMCID: PMC10354075
- DOI: 10.1038/s41467-023-40086-3
HCK induces macrophage activation to promote renal inflammation and fibrosis via suppression of autophagy
Abstract
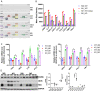
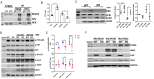
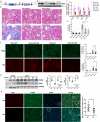
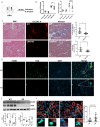
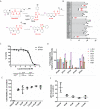
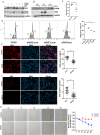
Renal inflammation and fibrosis are the common pathways leading to progressive chronic kidney disease (CKD). We previously identified hematopoietic cell kinase (HCK) as upregulated in human chronic allograft injury promoting kidney fibrosis; however, the cellular source and molecular mechanisms are unclear. Here, using immunostaining and single cell sequencing data, we show that HCK expression is highly enriched in pro-inflammatory macrophages in diseased kidneys. HCK-knockout (KO) or HCK-inhibitor decreases macrophage M1-like pro-inflammatory polarization, proliferation, and migration in RAW264.7 cells and bone marrow-derived macrophages (BMDM). We identify an interaction between HCK and ATG2A and CBL, two autophagy-related proteins, inhibiting autophagy flux in macrophages. In vivo, both global or myeloid cell specific HCK-KO attenuates renal inflammation and fibrosis with reduces macrophage numbers, pro-inflammatory polarization and migration into unilateral ureteral obstruction (UUO) kidneys and unilateral ischemia reperfusion injury (IRI) models. Finally, we developed a selective boron containing HCK inhibitor which can reduce macrophage pro-inflammatory activity, proliferation, and migration in vitro, and attenuate kidney fibrosis in the UUO mice. The current study elucidates mechanisms downstream of HCK regulating macrophage activation and polarization via autophagy in CKD and identifies that selective HCK inhibitors could be potentially developed as a new therapy for renal fibrosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

