Human blood neutrophils generate ROS through FcγR-signaling to mediate protection against febrile P. falciparum malaria
- PMID: 37463969
- PMCID: PMC10354059
- DOI: 10.1038/s42003-023-05118-0
Human blood neutrophils generate ROS through FcγR-signaling to mediate protection against febrile P. falciparum malaria
Abstract
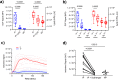
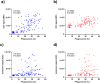
Blood phagocytes, such as neutrophils and monocytes, generate reactive oxygen species (ROS) as a part of host defense response against infections. We investigated the mechanism of Fcγ-Receptor (FcγR) mediated ROS production in these cells to understand how they contribute to anti-malarial immunity. Plasmodium falciparum merozoites opsonized with naturally occurring IgG triggered both intracellular and extracellular ROS generation in blood phagocytes, with neutrophils being the main contributors. Using specific inhibitors, we show that both FcγRIIIB and FcγRIIA acted synergistically to induce ROS production in neutrophils, and that NADPH oxidase 2 and the PI3K intracellular signal transduction pathway were involved in this process. High levels of neutrophil ROS were also associated with protection against febrile malaria in two geographically diverse malaria endemic regions from Ghana and India, stressing the importance of the cooperation between anti-malarial IgG and neutrophils in triggering ROS-mediated parasite killing as a mechanism for naturally acquired immunity against malaria.
© 2023. The Author(s).
Conflict of interest statement
S.S. is a co-founder of ProtExtent Biosolutions Pvt. Ltd., India. The remaining authors declare no competing interests.
Figures




Similar articles
-
Molecular and Functional Characterization of Fcγ Receptor IIIb-Ligand Interaction: Implications for Neutrophil-Mediated Immune Mechanisms in Malaria.Infect Immun. 2018 Jul 23;86(8):e00924-17. doi: 10.1128/IAI.00924-17. Print 2018 Aug. Infect Immun. 2018. PMID: 29784860 Free PMC article.
-
Neutrophils dominate in opsonic phagocytosis of P. falciparum blood-stage merozoites and protect against febrile malaria.Commun Biol. 2021 Aug 19;4(1):984. doi: 10.1038/s42003-021-02511-5. Commun Biol. 2021. PMID: 34413459 Free PMC article.
-
Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies.PLoS One. 2010 Mar 25;5(3):e9871. doi: 10.1371/journal.pone.0009871. PLoS One. 2010. PMID: 20360847 Free PMC article.
-
Clinical and parasitological studies on immunity to Plasmodium falciparum malaria in children.Scand J Infect Dis Suppl. 1996;102:1-53. Scand J Infect Dis Suppl. 1996. PMID: 9060051 Review.
-
Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance.Front Cell Infect Microbiol. 2017 Aug 25;7:373. doi: 10.3389/fcimb.2017.00373. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28890882 Free PMC article. Review.
Cited by
-
Elucidation of the Active Agents in a West African Ground Herbal Medicine Formulation That Elicit Antimalarial Activities in In Vitro and In Vivo Models.Molecules. 2024 Nov 29;29(23):5658. doi: 10.3390/molecules29235658. Molecules. 2024. PMID: 39683816 Free PMC article.
-
Reactive Oxygen Species: Role in Pathophysiology, and Mechanism of Endogenous and Dietary Antioxidants during Oxidative Stress.Chonnam Med J. 2025 Jan;61(1):32-45. doi: 10.4068/cmj.2025.61.1.32. Epub 2025 Jan 24. Chonnam Med J. 2025. PMID: 39958267 Free PMC article. Review.
-
A Non-Coding Fc Gamma Receptor Cis-Regulatory Variant within the 1q23 Gene Cluster Is Associated with Plasmodium falciparum Infection in Children Residing in Burkina Faso.Int J Mol Sci. 2023 Oct 28;24(21):15711. doi: 10.3390/ijms242115711. Int J Mol Sci. 2023. PMID: 37958695 Free PMC article.
-
FcRγIIA attenuates pathology of cutaneous leishmaniasis and modulates ITAMa/i balance.Parasit Vectors. 2024 Dec 18;17(1):517. doi: 10.1186/s13071-024-06593-y. Parasit Vectors. 2024. PMID: 39696675 Free PMC article.
-
Unveiling the Role of Selenium in Child Development: Impacts on Growth, Neurodevelopment and Immunity.J Clin Med. 2025 Feb 14;14(4):1274. doi: 10.3390/jcm14041274. J Clin Med. 2025. PMID: 40004804 Free PMC article. Review.
References
-
- WHO. World Malaria Report 2022. (2022).
-
- Healer, J., Chiu, C. Y. & Hansen, D. S. Mechanisms of naturally acquired immunity to P. falciparum and approaches to identify merozoite antigen targets. Parasitology145, 839–847 (2017). - PubMed
-
- McGregor IA, Carrington SP, Cohen S. Treatment of East African P. falciparum malaria with West African gammaglobulin. Trans. R. Soc. Trop. Med. Hyg. 1963;57:170–175. doi: 10.1016/0035-9203(63)90058-0. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

