Identification of circulating monocytes as producers of tuberculosis disease biomarker C1q
- PMID: 37464009
- PMCID: PMC10354225
- DOI: 10.1038/s41598-023-38889-x
Identification of circulating monocytes as producers of tuberculosis disease biomarker C1q
Abstract
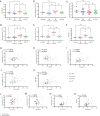
Tuberculosis (TB) is a prevalent disease causing an estimated 1.6 million deaths and 10.6 million new cases annually. Discriminating TB disease from differential diagnoses can be complex, particularly in the field. Increased levels of complement component C1q in serum have been identified as a specific and accessible biomarker for TB disease but the source of C1q in circulation has not been identified. Here, data and samples previously collected from human cohorts, a clinical trial and a non-human primate study were used to identify cells producing C1q in circulation. Cell subset frequencies were correlated with serum C1q levels and combined with single cell RNA sequencing and flow cytometry analyses. This identified monocytes as C1q producers in circulation, with a pronounced expression of C1q in classical and intermediate monocytes and variable expression in non-classical monocytes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
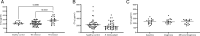


References
-
- Goletti D, Delogu G, Matteelli A, Migliori GB. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int. J. Infect. Dis. 2022;124(Suppl 1):S12–S19. doi: 10.1016/j.ijid.2022.02.047. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

