Calretinin-expressing islet cells are a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord
- PMID: 37464016
- PMCID: PMC10354228
- DOI: 10.1038/s41598-023-38605-9
Calretinin-expressing islet cells are a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord
Abstract
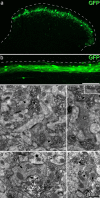
Unmyelinated non-peptidergic nociceptors (NP afferents) arborise in lamina II of the spinal cord and receive GABAergic axoaxonic synapses, which mediate presynaptic inhibition. However, until now the source of this axoaxonic synaptic input was not known. Here we provide evidence that it originates from a population of inhibitory calretinin-expressing interneurons (iCRs), which correspond to lamina II islet cells. The NP afferents can be assigned to 3 functionally distinct classes (NP1-3). NP1 afferents have been implicated in pathological pain states, while NP2 and NP3 afferents also function as pruritoceptors. Our findings suggest that all 3 of these afferent types innervate iCRs and receive axoaxonic synapses from them, providing feedback inhibition of NP input. The iCRs also form axodendritic synapses, and their targets include cells that are themselves innervated by the NP afferents, thus allowing for feedforward inhibition. The iCRs are therefore ideally placed to control the input from non-peptidergic nociceptors and pruritoceptors to other dorsal horn neurons, and thus represent a potential therapeutic target for the treatment of chronic pain and itch.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

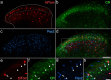
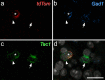



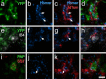
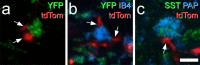


Update of
-
Calretinin-expressing islet cells: a source of pre- and post-synaptic inhibition of non-peptidergic nociceptor input to the mouse spinal cord.bioRxiv [Preprint]. 2023 Jun 5:2023.06.01.543241. doi: 10.1101/2023.06.01.543241. bioRxiv. 2023. Update in: Sci Rep. 2023 Jul 18;13(1):11561. doi: 10.1038/s41598-023-38605-9. PMID: 37333120 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

