Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses
- PMID: 37464041
- PMCID: PMC10839245
- DOI: 10.1038/s41591-023-02352-1
Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses
Abstract
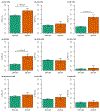
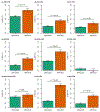
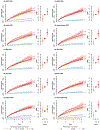
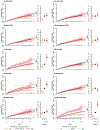
Depression is a common psychiatric disorder and a leading cause of disability worldwide. Here we conducted a genome-wide association study meta-analysis of six datasets, including >1.3 million individuals (371,184 with depression) and identified 243 risk loci. Overall, 64 loci were new, including genes encoding glutamate and GABA receptors, which are targets for antidepressant drugs. Intersection with functional genomics data prioritized likely causal genes and revealed new enrichment of prenatal GABAergic neurons, astrocytes and oligodendrocyte lineages. We found depression to be highly polygenic, with ~11,700 variants explaining 90% of the single-nucleotide polymorphism heritability, estimating that >95% of risk variants for other psychiatric disorders (anxiety, schizophrenia, bipolar disorder and attention deficit hyperactivity disorder) were influencing depression risk when both concordant and discordant variants were considered, and nearly all depression risk variants influenced educational attainment. Additionally, depression genetic risk was associated with impaired complex cognition domains. We dissected the genetic and clinical heterogeneity, revealing distinct polygenic architectures across subgroups of depression and demonstrating significantly increased absolute risks for recurrence and psychiatric comorbidity among cases of depression with the highest polygenic burden, with considerable sex differences. The risks were up to 5- and 32-fold higher than cases with the lowest polygenic burden and the background population, respectively. These results deepen the understanding of the biology underlying depression, its disease progression and inform precision medicine approaches to treatment.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
B.J.V. is a member of the advisory board for Allelica. D.D. has received a speaker fee from Takeda. B.W., T.T., H.S. and K.S. are employed at deCODE/Amgen. M.J.D. is a founder of Maze Therapeutics and on the Scientific Advisory Board of RBNC Therapeutics. The other authors declare no competing interests.
Figures



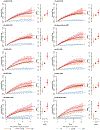
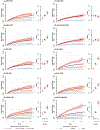
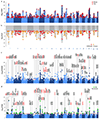


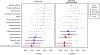
Comment in
-
Genetic insights into depression.Nat Genet. 2023 Aug;55(8):1253. doi: 10.1038/s41588-023-01482-z. Nat Genet. 2023. PMID: 37558886 No abstract available.
References
Publication types
MeSH terms
Associated data
Grants and funding
- R01 MH124851/MH/NIMH NIH HHS/United States
- U01 MH116442/MH/NIMH NIH HHS/United States
- IK2 BX005058/BX/BLRD VA/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 DK125246/DK/NIDDK NIH HHS/United States
- T32 MH087004/MH/NIMH NIH HHS/United States
- S10 OD030463/OD/NIH HHS/United States
- R01 AI116442/AI/NIAID NIH HHS/United States
- K08 MH122911/MH/NIMH NIH HHS/United States
- S10 OD026880/OD/NIH HHS/United States
- R01 MH109677/MH/NIMH NIH HHS/United States
- R01 AG067025/AG/NIA NIH HHS/United States
- U01 MH109514/MH/NIMH NIH HHS/United States
- R01 DK109677/DK/NIDDK NIH HHS/United States
- R01 MH125246/MH/NIMH NIH HHS/United States

