Piper nigrum extract suppresses tumor growth and enhances the antitumor immune response in murine models of breast cancer and melanoma
- PMID: 37464192
- PMCID: PMC10491708
- DOI: 10.1007/s00262-023-03487-3
Piper nigrum extract suppresses tumor growth and enhances the antitumor immune response in murine models of breast cancer and melanoma
Abstract
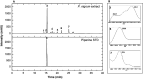
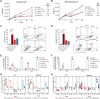
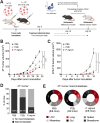
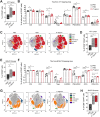
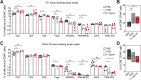
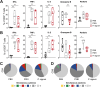
Although the antitumor effect of P. nigrum has been widely studied, research related to its possible immunomodulatory effects is relatively scarce. Here, the antitumor and immunomodulatory activity of an ethanolic extract of P. nigrum were evaluated in the murine models of 4T1 breast cancer and B16-F10 melanoma. In vitro evaluations showed that the P. nigrum extract has cytotoxic activity, induces apoptotic cell death, and has a pro-oxidant effect in both cell lines, but it regulates glucose uptake differently in both lines, decreasing it in 4T1 but not in B16-F10. P. nigrum extract significantly reduced tumor size in both models and decreased the occurrence of macrometastases in 4T1 model. Evaluation of immune subpopulations by flow cytometry revealed that the P. nigrum extract significantly increases the frequency of dendritic cells and activated CD8+ T cells and decreases the frequency of myeloid-derived suppressor like cells and Tregs in the tumor microenvironment of both models but with different dynamics. Our findings strongly suggest that the P. nigrum extract exerts immunomodulatory functions, slightly related to the modulation of cellular energy metabolism, which could ultimately contribute to the promising antitumor effect of P. nigrum.
Keywords: Antitumor; Breast cancer; Immunomodulation; Melanoma; Piper nigrum; Plant extracts.
© 2023. The Author(s).
Conflict of interest statement
S.F. and C.U. are inventors of a granted patent related to P2Et. SF and CU are partners of the DreemBio company who was a licensee of related patents. The rest of the authors declare no competing interests.
Figures

References
-
- Asprey G, Thornton P. Medical plants of Jamaica. The West Indian Med J. 1954;3:17–41. - PubMed
-
- Kirtikar K, Basu B (1935) Indian medicinal plants. Indian Medicinal Plants
-
- Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA. Biological role of Piper nigrum L. (Black pepper): a review. Asian Pac J Trop Biomed. 2012;2:S1945–S1953. doi: 10.1016/S2221-1691(12)60524-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

