New medicines for spontaneous preterm birth prevention and preterm labour management: landscape analysis of the medicine development pipeline
- PMID: 37464260
- PMCID: PMC10354994
- DOI: 10.1186/s12884-023-05842-9
New medicines for spontaneous preterm birth prevention and preterm labour management: landscape analysis of the medicine development pipeline
Abstract
Background: There are few medicines in clinical use for managing preterm labor or preventing spontaneous preterm birth from occurring. We previously developed two target product profiles (TPPs) for medicines to prevent spontaneous preterm birth and manage preterm labor. The objectives of this study were to 1) analyse the research and development pipeline of medicines for preterm birth and 2) compare these medicines to target product profiles for spontaneous preterm birth to identify the most promising candidates.
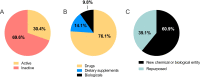
Methods: Adis Insight, Pharmaprojects, WHO international clinical trials registry platform (ICTRP), PubMed and grant databases were searched to identify candidate medicines (including drugs, dietary supplements and biologics) and populate the Accelerating Innovations for Mothers (AIM) database. This database was screened for all candidates that have been investigated for preterm birth. Candidates in clinical development were ranked against criteria from TPPs, and classified as high, medium or low potential. Preclinical candidates were categorised by product type, archetype and medicine subclass.
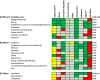
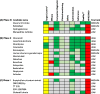
Results: The AIM database identified 178 candidates. Of the 71 candidates in clinical development, ten were deemed high potential (Prevention: Omega-3 fatty acid, aspirin, vaginal progesterone, oral progesterone, L-arginine, and selenium; Treatment: nicorandil, isosorbide dinitrate, nicardipine and celecoxib) and seven were medium potential (Prevention: pravastatin and lactoferrin; Treatment: glyceryl trinitrate, retosiban, relcovaptan, human chorionic gonadotropin and Bryophyllum pinnatum extract). 107 candidates were in preclinical development.
Conclusions: This analysis provides a drug-agnostic approach to assessing the potential of candidate medicines for spontaneous preterm birth. Research should be prioritised for high-potential candidates that are most likely to meet the real world needs of women, babies, and health care professionals.
Keywords: Aspirin; Celecoxib; Drug development; Isosorbide dinitrate; L-arginine; Nicardipine; Nicorandil; Omega-3 fatty acid; Oral progesterone; Preterm labour; Selenium; Tocolytics; Vaginal progesterone.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Systematic evaluation of the pre-eclampsia drugs, dietary supplements and biologicals pipeline using target product profiles.BMC Med. 2022 Nov 4;20(1):393. doi: 10.1186/s12916-022-02582-z. BMC Med. 2022. PMID: 36329468 Free PMC article.
-
Expert consensus on novel medicines to prevent preterm birth and manage preterm labour: Target product profiles.BJOG. 2024 Jan;131(1):71-80. doi: 10.1111/1471-0528.17314. Epub 2022 Oct 27. BJOG. 2024. PMID: 36209501
-
Analysis of a maternal health medicines pipeline database 2000-2021: New candidates for the prevention and treatment of fetal growth restriction.BJOG. 2023 May;130(6):653-663. doi: 10.1111/1471-0528.17392. Epub 2023 Feb 5. BJOG. 2023. PMID: 36655375
-
Omega-3 fatty acid addition during pregnancy.Cochrane Database Syst Rev. 2018 Nov 15;11(11):CD003402. doi: 10.1002/14651858.CD003402.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480773 Free PMC article.
-
The use of progesterone and other progestational agents to prevent spontaneous preterm labour and preterm birth.Expert Opin Pharmacother. 2009 Apr;10(6):1007-16. doi: 10.1517/14656560902851403. Expert Opin Pharmacother. 2009. PMID: 19351272 Review.
Cited by
-
Evaluation of a two-tier preterm birth prevention service in a tertiary hospital in the United Kingdom: a retrospective cohort study.BMC Pregnancy Childbirth. 2025 Apr 15;25(1):452. doi: 10.1186/s12884-025-07538-8. BMC Pregnancy Childbirth. 2025. PMID: 40234805 Free PMC article.
-
Cooperation of aquaporin 5 and the adrenergic system in the initiation of birth in rat model.Heliyon. 2024 Sep 3;10(17):e37329. doi: 10.1016/j.heliyon.2024.e37329. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39296125 Free PMC article.
-
A historical narrative review through the field of tocolysis in threatened preterm birth.Eur J Obstet Gynecol Reprod Biol X. 2024 Apr 29;22:100313. doi: 10.1016/j.eurox.2024.100313. eCollection 2024 Jun. Eur J Obstet Gynecol Reprod Biol X. 2024. PMID: 38736527 Free PMC article. Review.
-
The drug drought in maternal health: an ongoing predicament.Lancet Glob Health. 2024 Jul;12(7):e1174-e1183. doi: 10.1016/S2214-109X(24)00144-X. Lancet Glob Health. 2024. PMID: 38876763 Free PMC article. Review.
-
Maternal gut microbiome interventions to improve maternal and perinatal health outcomes: Target product profile expert consensus and pipeline analysis.PLoS One. 2025 Jul 2;20(7):e0321543. doi: 10.1371/journal.pone.0321543. eCollection 2025. PLoS One. 2025. PMID: 40601677 Free PMC article.
References
-
- Hug L, Alexander M, You D, Alkema L, Estimation UNI-aGfCM National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. 2019;7(6):e710–e20. doi: 10.1016/S2214-109X(19)30163-9. - DOI - PMC - PubMed
-
- Morisaki N, Togoobaatar G, Vogel JP, Souza JP, Rowland Hogue CJ, Jayaratne K, et al. Risk factors for spontaneous and provider-initiated preterm delivery in high and low Human Development Index countries: a secondary analysis of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG . 2014;121(Suppl 1):101–109. doi: 10.1111/1471-0528.12631. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

