Alterations in iron content, iron-regulatory proteins and behaviour without tau pathology at one year following repetitive mild traumatic brain injury
- PMID: 37464280
- PMCID: PMC10353227
- DOI: 10.1186/s40478-023-01603-z
Alterations in iron content, iron-regulatory proteins and behaviour without tau pathology at one year following repetitive mild traumatic brain injury
Abstract
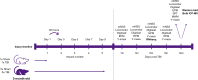
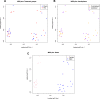
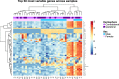
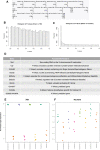
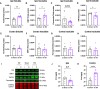
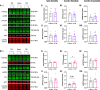
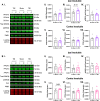
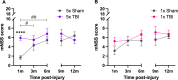
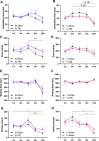
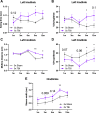
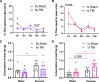
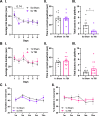
Repetitive mild traumatic brain injury (r-mTBI) has increasingly become recognised as a risk factor for the development of neurodegenerative diseases, many of which are characterised by tau pathology, metal dyshomeostasis and behavioural impairments. We aimed to characterise the status of tau and the involvement of iron dyshomeostasis in repetitive controlled cortical impact injury (5 impacts, 48 h apart) in 3-month-old C57Bl6 mice at the chronic (12-month) time point. We performed a battery of behavioural tests, characterised the status of neurodegeneration-associated proteins (tau and tau-regulatory proteins, amyloid precursor protein and iron-regulatory proteins) via western blot; and metal levels using bulk inductively coupled plasma-mass spectrometry (ICP-MS). We report significant changes in various ipsilateral iron-regulatory proteins following five but not a single injury, and significant increases in contralateral iron, zinc and copper levels following five impacts. There was no evidence of tau pathology or changes in tau-regulatory proteins following five impacts, although some changes were observed following a single injury. Five impacts resulted in significant gait deficits, mild anhedonia and mild cognitive deficits at 9-12 months post-injury, effects not seen following a single injury. To the best of our knowledge, we are the first to describe chronic changes in metals and iron-regulatory proteins in a mouse model of r-mTBI, providing a strong indication towards an overall increase in brain iron levels (and other metals) in the chronic phase following r-mTBI. These results bring to question the relevance of tau and highlight the involvement of iron dysregulation in the development and/or progression of neurodegeneration following injury, which may lead to new therapeutic approaches in the future.
Keywords: Iron-regulatory proteins; Metal dyshomeostasis; Neurodegeneration; Repetitive mild traumatic brain injury; Tau phosphorylation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that no financial and non-financial competing interests exist.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

