Comorbid asthma in patients with chronic rhinosinusitis with nasal polyps: did dupilumab make a difference?
- PMID: 37464395
- PMCID: PMC10354942
- DOI: 10.1186/s12890-023-02556-8
Comorbid asthma in patients with chronic rhinosinusitis with nasal polyps: did dupilumab make a difference?
Abstract
Background: The clinical heterogeneity of chronic rhinosinusitis (CRS) and bronchial asthma is attributable to different underlying inflammatory profiles. However, the similarity between CRS with nasal polyps (CRSwNP) and type-2 asthma pathophysiology speculates that one biological therapy could affect both comorbidities. Despite dupilumab, a monoclonal antibody that targets IL-4α and IL-13 receptors, being used in patients with nasal polyps and severe asthma, real-life data about its efficacy in improving the quality of life and patient symptoms is still lacking. This study's primary objective was to evaluate dupilumab treatment's effect on the frequency of olfactory symptoms and health-related quality of life tests as measured by the Sino-nasal outcome test (SNOT-22) in patients with NP. The secondary objective was the effect of dupilumab on asthma symptom control as measured by the asthma control test (ACT).
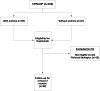
Methods: A prospective study was conducted of 166 patients with CRSwNP, with or without asthma. The following variables were collected at baseline and after at least six months of continuous dupilumab therapy; SNOT-22, olfactory symptoms frequency, and ACT score.
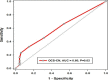
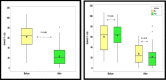
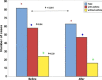
Results: Asthma prevalence in patients with CRSwNP was high (59.63%), and being female with a history of frequent use of oral corticosteroid (OCS) courses and repeated unsuccessful nasal and para-nasal surgeries for polyposis increased the likelihood of having underlying asthma by 2, 1 and 4 times more, respectively. Additionally, being asthmatic required a longer duration of dupilumab treatment. However, both the health-related quality of life and olfactory symptoms improved equally in both groups.
Conclusion: Even with associated comorbid asthma in patients with CRSwNP, treatment with dupilumab could improve the quality of life, olfactory symptoms, and asthma symptom control.
Keywords: Asthma; Asthma control test; Chronic rhinosinusitis; Dupilumab; Nasal polyps; Sinonasal outcome test.
© 2023. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest. Each author has revised and approved the final version of the manuscript independently.
Figures


References
-
- Fokkens W, Van Der Lans R, Reitsma S. Dupilumab for the treatment of chronic rhinosinusitis with nasal polyposis. Expert Opin Biol Ther [Internet]. 2021 May 4;21(5):575–85. Available from: https://www.tandfonline.com/doi/full/10.1080/14712598.2021.1901881. - PubMed
-
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A et al. Chronic rhinosinusitis in Europe - an underestimated disease. A GA2LEN study. Allergy [Internet]. 2011 Sep;66(9):1216–23. Available from: https://onlinelibrary.wiley.com/doi/10.1111/j.1398-9995.2011.02646.x. - PubMed
-
- Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy [Internet]. 2017 Feb;72(2):274–81. Available from: https://onlinelibrary.wiley.com/doi/10.1111/all.13042. - PMC - PubMed
-
- Obaseki D, Potts J, Joos G, Baelum J, Haahtela T, Ahlström M et al. The relation of airway obstruction to asthma, chronic rhinosinusitis and age: results from a population survey of adults. Allergy [Internet]. 2014 Sep 19;69(9):1205–14. Available from: https://onlinelibrary.wiley.com/doi/10.1111/all.12447. - PMC - PubMed
-
- Sundaresan AS, Hirsch AG, Storm M, Tan BK, Kennedy TL, Greene JS et al. Occupational and environmental risk factors for chronic rhinosinusitis: a systematic review. Int Forum Allergy Rhinol [Internet]. 2015 Nov;5(11):996–1003. Available from: https://onlinelibrary.wiley.com/doi/10.1002/alr.21573. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

