The Relationship of Astrocytes and Microglia with Different Stages of Ischemic Stroke
- PMID: 37464832
- PMCID: PMC10616922
- DOI: 10.2174/1570159X21666230718104634
The Relationship of Astrocytes and Microglia with Different Stages of Ischemic Stroke
Abstract
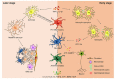
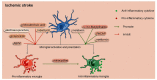
Ischemic stroke is the predominant cause of severe morbidity and mortality worldwide. Post-stroke neuroinflammation has recently received increasing attention with the aim of providing a new effective treatment strategy for ischemic stroke. Microglia and astrocytes are major components of the innate immune system of the central nervous system. They can be involved in all phases of ischemic stroke, from the early stage, contributing to the first wave of neuronal cell death, to the late stage involving phagocytosis and repair. In the early stage of ischemic stroke, a vicious cycle exists between the activation of microglia and astrocytes (through astrocytic connexin 43 hemichannels), aggravating neuroinflammatory injury post-stroke. However, in the late stage of ischemic stroke, repeatedly activated microglia can induce the formation of glial scars by triggering reactive astrogliosis in the peri-infarct regions, which may limit the movement of activated microglia in reverse and restrict the diffusion of inflammation to healthy brain tissues, alleviating the neuroinflammatory injury poststroke. In this review, we elucidated the various roles of astrocytes and microglia and summarized their relationship with neuroinflammation. We also examined how astrocytes and microglia influence each other at different stages of ischemic stroke. Several potential therapeutic approaches targeting astrocytes and microglia in ischemic stroke have been reviewed. Understanding the details of astrocytemicroglia interaction processes will contribute to a better understanding of the mechanisms underlying ischemic stroke, contributing to the identification of new therapeutic interventions.
Keywords: Ischemic stroke; astrocyte-microglia interaction; astrocytes; microglia; neuroinflammation; stroke treatment.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
Similar articles
-
Glial Cells: Role of the Immune Response in Ischemic Stroke.Front Immunol. 2020 Feb 26;11:294. doi: 10.3389/fimmu.2020.00294. eCollection 2020. Front Immunol. 2020. PMID: 32174916 Free PMC article. Review.
-
Flavonoids and ischemic stroke-induced neuroinflammation: Focus on the glial cells.Biomed Pharmacother. 2024 Jan;170:115847. doi: 10.1016/j.biopha.2023.115847. Epub 2023 Nov 27. Biomed Pharmacother. 2024. PMID: 38016362 Review.
-
Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte.J Neuroinflammation. 2020 Sep 11;17(1):270. doi: 10.1186/s12974-020-01946-7. J Neuroinflammation. 2020. PMID: 32917229 Free PMC article.
-
Time-dependent dual effect of microglia in ischemic stroke.Neurochem Int. 2023 Oct;169:105584. doi: 10.1016/j.neuint.2023.105584. Epub 2023 Jul 15. Neurochem Int. 2023. PMID: 37454817 Review.
-
Activated microglia-derived macrophage-like cells exacerbate brain edema after ischemic stroke correlate with astrocytic expression of aquaporin-4 and interleukin-1 alpha release.Neurochem Int. 2020 Nov;140:104848. doi: 10.1016/j.neuint.2020.104848. Epub 2020 Sep 11. Neurochem Int. 2020. PMID: 32920036
Cited by
-
Effects of intermittent theta burst stimulation on the inflammatory response and cerebral blood flow in promoting neurovascular repair after ischemic stroke.Mol Brain. 2025 Jun 9;18(1):48. doi: 10.1186/s13041-025-01222-w. Mol Brain. 2025. PMID: 40490760 Free PMC article.
-
Human induced neural progenitor cells generated from three-dimensional aggregate-based culture significantly improve post-stroke recovery in tMCAO mice.Stem Cell Res Ther. 2025 Jun 20;16(1):312. doi: 10.1186/s13287-025-04433-z. Stem Cell Res Ther. 2025. PMID: 40542444 Free PMC article.
-
Neuroglia and immune cells play different roles in neuroinflammation and neuroimmune response in post-stroke neural injury and repair.Acta Pharmacol Sin. 2025 Aug 12. doi: 10.1038/s41401-025-01640-5. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40797113 Review.
-
Blood-brain barrier repair: potential and challenges of stem cells and exosomes in stroke treatment.Front Cell Neurosci. 2025 Apr 7;19:1536028. doi: 10.3389/fncel.2025.1536028. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40260076 Free PMC article. Review.
-
Pulmonary consequences of experimentally induced stroke: differences between global and focal cerebral ischemia.Front Physiol. 2024 Dec 12;15:1511638. doi: 10.3389/fphys.2024.1511638. eCollection 2024. Front Physiol. 2024. PMID: 39726861 Free PMC article.
References
-
- Timmis A., Townsend N., Gale C.P., Torbica A., Lettino M., Petersen S.E., Mossialos E.A., Maggioni A.P., Kazakiewicz D., May H.T., De Smedt D., Flather M., Zuhlke L., Beltrame J.F., Huculeci R., Tavazzi L., Hindricks G., Bax J., Casadei B., Achenbach S., Wright L., Vardas P., Mimoza L., Artan G., Aurel D., Chettibi M., Hammoudi N., Sisakian H., Pepoyan S., Metzler B., Siostrzonek P., Weidinger F., Jahangirov T., Aliyev F., Rustamova Y., Manak N., Mrochak A., Lancellotti P., Pasquet A., Claeys M. Kušljugić Z.; Dizdarević Hudić, L.; Smajić, E.; Tokmakova, M.P.; Gatzov, P.M.; Milicic, D.; Bergovec, M.; Christou, C.; Moustra, H.H.; Christodoulides, T.; Linhart, A.; Taborsky, M.; Hansen, H.S.; Holmvang, L.; Kristensen, S.D.; Abdelhamid, M.; Shokry, K.; Kampus, P.; Viigimaa, M.; Ryödi, E.; Niemelä, M.; Rissanen, T.T.; Le Heuzey, J-Y.; Gilard, M.; Aladashvili, A.; Gamkrelidze, A.; Kereselidze, M.; Zeiher, A.; Katus, H.; Bestehorn, K.; Tsioufis, C.; Goudevenos, J.; Csanádi, Z.; Becker, D.; Tóth, K.; Jóna, H.Þ.; Crowley, J.; Kearney, P.; Dalton, B.; Zahger, D.; Wolak, A.; Gabrielli, D.; Indolfi, C.; Urbinati, S.; Imantayeva, G.; Berkinbayev, S.; Bajraktari, G.; Ahmeti, A.; Berisha, G.; Erkin, M.; Saamay, A.; Erglis, A.; Bajare, I.; Jegere, S.; Mohammed, M.; Sarkis, A.; Saadeh, G.; Zvirblyte, R.; Sakalyte, G.; Slapikas, R.; Ellafi, K.; El Ghamari, F.; Banu, C.; Beissel, J.; Felice, T.; Buttigieg, S.C.; Xuereb, R.G.; Popovici, M.; Boskovic, A.; Rabrenovic, M.; Ztot, S.; Abir-Khalil, S.; van Rossum, A.C.; Mulder, B.J.M.; Elsendoorn, M.W.; Srbinovska-Kostovska, E.; Kostov, J.; Marjan, B.; Steigen, T.; Mjølstad, O.C.; Ponikowski, P.; Witkowski, A.; Jankowski, P.; Gil, V.M.; Mimoso, J.; Baptista, S.; Vinereanu, D.; Chioncel, O.; Popescu, B.A.; Shlyakhto, E.; Oganov, R.; Foscoli, M.; Zavatta, M.; Dikic, A.D.; Beleslin, B.; Radovanovic, M.R.; Hlivák, P.; Hatala, R.; Kaliská, G.; Kenda, M.; Fras, Z.; Anguita, M.; Cequier, Á.; Muñiz, J.; James, S.; Johansson, B.; Platonov, P.; Zellweger, M.J.; Pedrazzini, G.B.; Carballo, D.; Shebli, H.E.; Kabbani, S.; Abid, L.; Addad, F.; Bozkurt, E.; Kayıkçıoğlu, M.; Erol, M.K.; Kovalenko, V.; Nesukay, E.; Wragg, A.; Ludman, P.; Ray, S.; Kurbanov, R.; Boateng, D.; Daval, G.; de Benito, R.V.; Sebastiao, D.; de Courtelary, P.T.; Bardinet, I. European society of cardiology: Cardiovascular disease statistics 2019. Eur. Heart J. 2020;41(1):12–85. doi: 10.1093/eurheartj/ehz859. - DOI - PubMed
-
- Zini A. Reperfusion therapies in acute ischemic stroke. G. Ital. Cardiol. 2019;20(5):279–288. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

