Perilipin 3 promotes the formation of membrane domains enriched in diacylglycerol and lipid droplet biogenesis proteins
- PMID: 37465010
- PMCID: PMC10350540
- DOI: 10.3389/fcell.2023.1116491
Perilipin 3 promotes the formation of membrane domains enriched in diacylglycerol and lipid droplet biogenesis proteins
Abstract
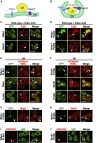
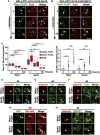
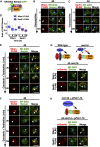
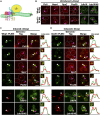
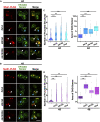
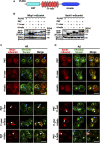
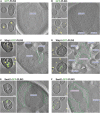
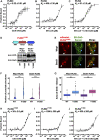
Lipid droplets (LDs) serve as intracellular stores of energy-rich neutral lipids. LDs form at discrete sites in the endoplasmic reticulum (ER) and they remain closely associated with the ER during lipogenic growth and lipolytic consumption. Their hydrophobic neutral lipid core is covered by a monolayer of phospholipids, which harbors a specific set of proteins. This LD surface is coated and stabilized by perilipins, a family of soluble proteins that specifically target LDs from the cytosol. We have previously used chimeric fusion proteins between perilipins and integral ER membrane proteins to test whether proteins that are anchored to the ER bilayer could be dragged onto the LD monolayer. Expression of these chimeric proteins induces repositioning of the ER membrane around LDs. Here, we test the properties of membrane-anchored perilipins in cells that lack LDs. Unexpectedly, membrane-anchored perilipins induce expansion and vesiculation of the perinuclear membrane resulting in the formation of crescent-shaped membrane domains that harbor LD-like properties. These domains are stained by LD-specific lipophilic dyes, harbor LD marker proteins, and they transform into nascent LDs upon induction of neutral lipid synthesis. These ER domains are enriched in diacylglycerol (DAG) and in ER proteins that are important for early steps of LD biogenesis, including seipin and Pex30. Formation of the domains in vivo depends on DAG levels, and we show that perilipin 3 (PLIN3) binds to liposomes containing DAG in vitro. Taken together, these observations indicate that perilipin not only serve to stabilize the surface of mature LDs but that they are likely to exert a more active role in early steps of LD biogenesis at ER subdomains enriched in DAG, seipin, and neutral lipid biosynthetic enzymes.
Keywords: Saccharomyces cerevisiae; diacylglycerol; endoplasmic reticulum; lipid droplets; perilipins; seipin; steryl esters; triacylglycerols.
Copyright © 2023 Khaddaj, Stribny, Cottier and Schneiter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Binding of perilipin 3 to membranes containing diacylglycerol is mediated by conserved residues within its PAT domain.J Biol Chem. 2023 Dec;299(12):105384. doi: 10.1016/j.jbc.2023.105384. Epub 2023 Oct 28. J Biol Chem. 2023. PMID: 37898398 Free PMC article.
-
Expression of oleosin and perilipins in yeast promotes formation of lipid droplets from the endoplasmic reticulum.J Cell Sci. 2013 Nov 15;126(Pt 22):5198-209. doi: 10.1242/jcs.131896. Epub 2013 Sep 4. J Cell Sci. 2013. PMID: 24006263
-
The surface of lipid droplets constitutes a barrier for endoplasmic reticulum-resident integral membrane proteins.J Cell Sci. 2022 Mar 1;135(5):jcs256206. doi: 10.1242/jcs.256206. Epub 2021 May 24. J Cell Sci. 2022. PMID: 34028531
-
Lipid droplet biogenesis: A mystery "unmixing"?Semin Cell Dev Biol. 2020 Dec;108:14-23. doi: 10.1016/j.semcdb.2020.03.001. Epub 2020 Mar 17. Semin Cell Dev Biol. 2020. PMID: 32192830 Review.
-
Born this way - Biogenesis of lipid droplets from specialized ER subdomains.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jan;1865(1):158448. doi: 10.1016/j.bbalip.2019.04.008. Epub 2019 Apr 24. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31028912 Review.
Cited by
-
Lipid droplet accumulation in microglia and their potential roles.Lipids Health Dis. 2025 Jun 14;24(1):215. doi: 10.1186/s12944-025-02633-3. Lipids Health Dis. 2025. PMID: 40514678 Free PMC article. Review.
-
Key enzymes involved in the utilization of fatty acids by Saccharomyces cerevisiae: a review.Front Microbiol. 2024 Jan 11;14:1294182. doi: 10.3389/fmicb.2023.1294182. eCollection 2023. Front Microbiol. 2024. PMID: 38274755 Free PMC article. Review.
-
Structural insights into perilipin 3 membrane association in response to diacylglycerol accumulation.Nat Commun. 2023 Jun 2;14(1):3204. doi: 10.1038/s41467-023-38725-w. Nat Commun. 2023. PMID: 37268630 Free PMC article.
-
Binding of perilipin 3 to membranes containing diacylglycerol is mediated by conserved residues within its PAT domain.J Biol Chem. 2023 Dec;299(12):105384. doi: 10.1016/j.jbc.2023.105384. Epub 2023 Oct 28. J Biol Chem. 2023. PMID: 37898398 Free PMC article.
-
Concept of lipid droplet biogenesis.Eur J Cell Biol. 2023 Dec;102(4):151362. doi: 10.1016/j.ejcb.2023.151362. Epub 2023 Sep 19. Eur J Cell Biol. 2023. PMID: 37742390 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

