The central role of DNA damage in immunosenescence
- PMID: 37465119
- PMCID: PMC10351018
- DOI: 10.3389/fragi.2023.1202152
The central role of DNA damage in immunosenescence
Abstract
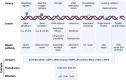
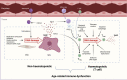
Ageing is the biggest risk factor for the development of multiple chronic diseases as well as increased infection susceptibility and severity of diseases such as influenza and COVID-19. This increased disease risk is linked to changes in immune function during ageing termed immunosenescence. Age-related loss of immune function, particularly in adaptive responses against pathogens and immunosurveillance against cancer, is accompanied by a paradoxical gain of function of some aspects of immunity such as elevated inflammation and increased incidence of autoimmunity. Of the many factors that contribute to immunosenescence, DNA damage is emerging as a key candidate. In this review, we discuss the evidence supporting the hypothesis that DNA damage may be a central driver of immunosenescence through senescence of both immune cells and cells of non-haematopoietic lineages. We explore why DNA damage accumulates during ageing in a major cell type, T cells, and how this may drive age-related immune dysfunction. We further propose that existing immunosenescence interventions may act, at least in part, by mitigating DNA damage and restoring DNA repair processes (which we term "genoprotection"). As such, we propose additional treatments on the basis of their evidence for genoprotection, and further suggest that this approach may provide a viable therapeutic strategy for improving immunity in older people.
Keywords: DNA damage; DNA repair; ageing; immunology; immunosenescence; senescence.
Copyright © 2023 Kell, Simon, Alsaleh and Cox.
Conflict of interest statement
AS consults for Oxford Healthspan, The Longevity Labs, and Calico. LC is Program Director (Dynamic Resilience) for non-profit Wellcome Leap and is co-director of UK Ageing Research Networks and BLAST ageing network (UKRI funded). LC holds voluntary roles with the All Party Parliamentary Group for Longevity (UK); European Geriatric Medicine Society special interest group in Ageing Biology; Clinical and Translational Theme panel, Biochemical Society (UK); and Medical Research Council Ageing Research Steering Group. GA and LK report no conflicts of interest.
Figures
Similar articles
-
Hallmarks and detection techniques of cellular senescence and cellular ageing in immune cells.Aging Cell. 2021 Feb;20(2):e13316. doi: 10.1111/acel.13316. Epub 2021 Feb 1. Aging Cell. 2021. PMID: 33524238 Free PMC article. Review.
-
Intracellular signalling pathways: targets to reverse immunosenescence.Clin Exp Immunol. 2017 Jan;187(1):35-43. doi: 10.1111/cei.12836. Epub 2016 Aug 3. Clin Exp Immunol. 2017. PMID: 27364690 Free PMC article. Review.
-
Interconnections between Inflammageing and Immunosenescence during Ageing.Cells. 2022 Jan 21;11(3):359. doi: 10.3390/cells11030359. Cells. 2022. PMID: 35159168 Free PMC article. Review.
-
An aged immune system drives senescence and ageing of solid organs.Nature. 2021 Jun;594(7861):100-105. doi: 10.1038/s41586-021-03547-7. Epub 2021 May 12. Nature. 2021. PMID: 33981041 Free PMC article.
-
Immunosenescence in atherosclerosis: A role for chronic viral infections.Front Immunol. 2022 Aug 17;13:945016. doi: 10.3389/fimmu.2022.945016. eCollection 2022. Front Immunol. 2022. PMID: 36059478 Free PMC article. Review.
Cited by
-
Sirt6 deficiency promotes senescence and age-associated intervertebral disc degeneration in mice.Bone Res. 2025 May 8;13(1):50. doi: 10.1038/s41413-025-00422-3. Bone Res. 2025. PMID: 40335469 Free PMC article.
-
Immunopharmacology of senescence: targeting the senescence-associated secretory phenotype (SASP)-a mechanism-based review.Inflammopharmacology. 2025 Aug;33(8):4291-4310. doi: 10.1007/s10787-025-01867-y. Epub 2025 Jul 26. Inflammopharmacology. 2025. PMID: 40715742 Review.
-
SIRT6 loss causes intervertebral disc degeneration in mice by promoting senescence and SASP status.bioRxiv [Preprint]. 2024 Sep 9:2024.09.09.612072. doi: 10.1101/2024.09.09.612072. bioRxiv. 2024. Update in: Bone Res. 2025 May 8;13(1):50. doi: 10.1038/s41413-025-00422-3. PMID: 39314282 Free PMC article. Updated. Preprint.
-
GRSF1 loss in THP-1 macrophages promotes senescence-associated transcription in neighboring fibroblasts.Sci Rep. 2025 Aug 14;15(1):29851. doi: 10.1038/s41598-025-11385-0. Sci Rep. 2025. PMID: 40813599 Free PMC article.
-
ERCC1 which affects lipids metabolism and actin dynamics in coal workers' pneumoconiosis is a candidate biomarker for early warning and diagnosis.PLoS One. 2024 Sep 16;19(9):e0308082. doi: 10.1371/journal.pone.0308082. eCollection 2024. PLoS One. 2024. PMID: 39283905 Free PMC article.
References
-
- Alimbetov D., Davis T., Brook A. J., Cox L. S., Faragher R. G., Nurgozhin T., et al. (2016). Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 17, 305–315. 10.1007/s10522-015-9610-z - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

