Natural antioxidants that act against Alzheimer's disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action
- PMID: 37465125
- PMCID: PMC10351420
- DOI: 10.3389/fendo.2023.1217730
Natural antioxidants that act against Alzheimer's disease through modulation of the NRF2 pathway: a focus on their molecular mechanisms of action
Abstract
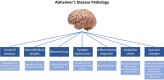
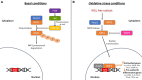
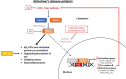
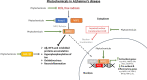
Characterized by a complex pathophysiology that includes the intraneuronal formation of neurofibrillary tangles and the extracellular deposition of β-amyloid plaques, Alzheimer's disease (AD) is a terminal neurodegenerative disease that causes dementia in older adults. Oxidative stress in the brain is considered as one of the contributing factors to the pathogenesis of AD, and thus, antioxidants have attracted much interest as potential therapeutic agents against the disorder. Natural antioxidants are typically characterized by low acute and chronic toxicity, which facilitates their potential therapeutic application. One important molecular target for the beneficial effects of natural antioxidants is the nuclear factor erythroid-derived 2-related factor 2 (NFE2L2/NRF2). NRF2 is a key transcription factor that orchestrates the cellular antioxidant response through regulating the expression of oxidative stress-related genes harboring the antioxidant response element (ARE) in their promoters. Indeed, in the case of excessive oxidative damage, NRF2 migrates to the nucleus and binds to ARE, activating the transcription of antioxidant protector genes. There is increasing evidence that NRF2 is implicated in AD pathology through dysfunction and altered localization, which renders it as a potential therapeutic target for AD. Thus, this review summarizes the most recent (2018-2023) advances on the NRF2-modulating activity of natural antioxidants observed in vitro and in AD animal models. This information will help elucidate the molecular mechanisms governing the antioxidant activity of such phytochemicals to highlight their therapeutic potential against common neurodegenerative diseases, such as AD.
Keywords: Alzheimer’s disease; NRF2; antioxidants; neurodegenerative diseases; neuroprotection; oxidative stress; reactive oxygen species.
Copyright © 2023 Sidiropoulou, Metaxas and Kourti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Impacts of oxidants and antioxidants on the emergence and progression of Alzheimer's disease.Neurochem Int. 2022 Feb;153:105268. doi: 10.1016/j.neuint.2021.105268. Epub 2021 Dec 23. Neurochem Int. 2022. PMID: 34954260 Review.
-
Mini-GAGR, an intranasally applied polysaccharide, activates the neuronal Nrf2-mediated antioxidant defense system.J Biol Chem. 2018 Nov 23;293(47):18242-18269. doi: 10.1074/jbc.RA117.001245. Epub 2018 Oct 3. J Biol Chem. 2018. PMID: 30282635 Free PMC article.
-
Counteracting role of nuclear factor erythroid 2-related factor 2 pathway in Alzheimer's disease.Biomed Pharmacother. 2020 Sep;129:110373. doi: 10.1016/j.biopha.2020.110373. Epub 2020 Jun 27. Biomed Pharmacother. 2020. PMID: 32603894 Review.
-
Role of Nrf2 in Synaptic Plasticity and Memory in Alzheimer's Disease.Cells. 2021 Jul 25;10(8):1884. doi: 10.3390/cells10081884. Cells. 2021. PMID: 34440653 Free PMC article. Review.
-
Therapeutic Approaches to Alzheimer's Disease Through Modulation of NRF2.Neuromolecular Med. 2019 Mar;21(1):1-11. doi: 10.1007/s12017-018-08523-5. Epub 2019 Jan 7. Neuromolecular Med. 2019. PMID: 30617737 Review.
Cited by
-
The role of Nrf2 signaling pathways in nerve damage repair.Toxicol Res (Camb). 2024 May 23;13(3):tfae080. doi: 10.1093/toxres/tfae080. eCollection 2024 Jun. Toxicol Res (Camb). 2024. PMID: 38799411 Free PMC article. Review.
-
Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis.Antioxidants (Basel). 2024 Mar 25;13(4):393. doi: 10.3390/antiox13040393. Antioxidants (Basel). 2024. PMID: 38671841 Free PMC article. Review.
-
A novel ferroptosis inhibitor, Thonningianin A, improves Alzheimer's disease by activating GPX4.Theranostics. 2024 Sep 23;14(16):6161-6184. doi: 10.7150/thno.98172. eCollection 2024. Theranostics. 2024. PMID: 39431016 Free PMC article.
-
Molecular targets and mechanisms of Sijunzi decoction in the treatment of Parkinson's disease: evidence from network pharmacology, molecular docking, molecular dynamics simulation, and experimental validation.Front Pharmacol. 2024 Nov 26;15:1487474. doi: 10.3389/fphar.2024.1487474. eCollection 2024. Front Pharmacol. 2024. PMID: 39660000 Free PMC article.
-
Korean black ginseng extract alleviates Alzheimer's disease-related cognitive impairment by activating the Nrf2/HO-1 pathway and suppressing the p38 MAPK/NF-κB/STAT3 pathways and NLRP3 inflammasome via TLR2 and TLR4 modulation.J Ginseng Res. 2025 May;49(3):294-305. doi: 10.1016/j.jgr.2025.02.002. Epub 2025 Feb 24. J Ginseng Res. 2025. PMID: 40453349 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

