Recommendations for patient involvement in health technology assessment in Central and Eastern European countries
- PMID: 37465169
- PMCID: PMC10350487
- DOI: 10.3389/fpubh.2023.1176200
Recommendations for patient involvement in health technology assessment in Central and Eastern European countries
Abstract
Introduction: Meaningful patient involvement in health technology assessment (HTA) is essential in ensuring that the interests of the affected patient population, their families, and the general public are accurately reflected in coverage and reimbursement decisions. Central and Eastern European (CEE) countries are generally at less advanced stages of implementing HTA, which is particularly true for patient involvement activities. As part of the Horizon2020 HTx project, this research aimed to form recommendations for critical barriers to patient involvement in HTA in CEE countries.
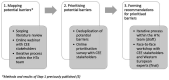
Methods: Built on previous research findings on potential barriers, a prioritisation survey was conducted online with CEE stakeholders. Recommendations for prioritised barriers were formed through a face-to-face workshop by CEE stakeholders and HTx experts.
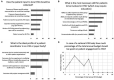
Results: A total of 105 stakeholders from 13 CEE countries completed the prioritisation survey and identified 12 of the 22 potential barriers as highly important. The workshop had 36 participants representing 9 CEE countries, and 5 Western European countries coming together to discuss solutions in order to form recommendations based on best practices, real-life experience, and transferability aspects. Stakeholder groups involved in both phases included HTA organisation representatives, payers, patients, caregivers, patient organisation representatives, patient experts, health care providers, academic and non-academic researchers, health care consultants and health technology manufacturers/providers. As a result, 12 recommendations were formed specified to the CEE region's context, but potentially useful for a broader geographic audience.
Conclusion: In this paper, we present 12 recommendations for meaningful, systematic, and sustainable patient involvement in HTA in CEE countries. Our hope is that engaging more than a hundred CEE stakeholders in the study helped to spread awareness of the importance and potential of patient involvement and that the resulting recommendations provide tangible steps for the way forward. Future studies shall focus on country-specific case studies of the implemented recommendations.
Keywords: Central and Eastern Europe countries; barrier prioritisation; decision-making; health technology assessment; patient involvement; recommendations; reimbursement; stakeholder perspectives.
Copyright © 2023 Jakab, Dimitrova, Houÿez, Bereczky, Fövényes, Maravic, Belina, Andriciuc, Tóth, Piniazhko, Hren, Gutierrez-Ibarluzea, Czech, Tesar, Niewada, Lorenzovici, Kamusheva, Manova, Savova, Mitkova, Tachkov, Németh, Petykó, Dawoud, Delnoij, Knies, Goettsch and Kaló.
Conflict of interest statement
Patient involvement should be open to all and non-discriminative on the grounds of previous experience and presumed support time needed. There are multiple initiatives locally and internationally aiming to ease patient recruitment with education, coordination and/or databases. We recommend organisations leading these initiatives to come together and join forces on the base of commonly agreed principles. We also recommend local HTA/payer organisations to set up their open call for local patients, carers, patient advocates, patient experts and patient organisation representatives to be able to express interest. We suggest to actively promote this opportunity to harder to reach patient communities and a periodical revision of the registry. Regarding ethical and compliance issues, a clear policy on financial and other conflicts of interests (how interests are declared, assessed and addressed) should be in place. Those registered should complete a declaration of interest form both on personal and organisational level conflict of interests and update it periodically. It should be clarified what kind of involvement with industry (e.g., attending a single advisory board meeting with a company versus only in case of direct conflict of interest) would impose restrictions on how a person can be involved in the HTA and decision-making process. We argue that in situations where patient experts and/or patient organisation representatives are difficult to identify, a softer approach should be taken and special measures could be proposed, equivalent to the “expert witness” status at the European Medicines Agency (EMA). Expert witnesses can be heard or participate in the deliberations but are not allowed to take part in the vote. However, the consequences of not being transparent with potential conflict of interests should be serious and communicated clearly from the beginning.KrT, RH, ZP, BN, and ZK were employed by Syreon Research Institute. At the time of the study IJ was the President of the European Patients’ Forum Youth Group, a Board of Trustees member at the EUPATI Foundation and employed by Syreon Research Institute. MN is the founder and co-owner of HealthQuest, a health technology assessment and market access consulting company. DaD is a Trustee of Thrombosis UK. TB was employed by Patvocates GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Potential Barriers of Patient Involvement in Health Technology Assessment in Central and Eastern European Countries.Front Public Health. 2022 Jul 28;10:922708. doi: 10.3389/fpubh.2022.922708. eCollection 2022. Front Public Health. 2022. PMID: 35968493 Free PMC article.
-
Recommendations to overcome barriers to the use of artificial intelligence-driven evidence in health technology assessment.Front Public Health. 2023 Apr 26;11:1088121. doi: 10.3389/fpubh.2023.1088121. eCollection 2023. Front Public Health. 2023. PMID: 37181704 Free PMC article.
-
Quo Vadis HTA for Medical Devices in Central and Eastern Europe? Recommendations to Address Methodological Challenges.Front Public Health. 2021 Jan 8;8:612410. doi: 10.3389/fpubh.2020.612410. eCollection 2020. Front Public Health. 2021. PMID: 33490024 Free PMC article.
-
Guidance on using real-world evidence from Western Europe in Central and Eastern European health policy decision making.J Comp Eff Res. 2023 Apr;12(4):e220157. doi: 10.57264/cer-2022-0157. Epub 2023 Mar 2. J Comp Eff Res. 2023. PMID: 36861458 Free PMC article. Review.
-
The use of non-economic criteria in pricing and reimbursement decisions in Central and Eastern Europe: issues, trends and recommendations.Expert Rev Pharmacoecon Outcomes Res. 2016 Aug;16(4):483-8. doi: 10.1080/14737167.2016.1215917. Expert Rev Pharmacoecon Outcomes Res. 2016. PMID: 27467881 Review.
Cited by
-
Involving Patients in Hospital-Based Health Technology Assessment of Innovative Medical Devices: Adapting to a Specific Local Context and Lessons Learned From the Assessment of an Ex Vivo Perfusion System of Human Donor Hearts.Health Expect. 2024 Dec;27(6):e70119. doi: 10.1111/hex.70119. Health Expect. 2024. PMID: 39660693 Free PMC article.
-
From Innovator Result-driven to Multi-actor Impact-oriented Public-Private Partnerships: Integrating the Patient Perspective.Handb Exp Pharmacol. 2024;286:137-168. doi: 10.1007/164_2024_730. Handb Exp Pharmacol. 2024. PMID: 39235487 Review.
-
Preferences for public health insurance coverage of new anticancer drugs: a discrete choice experiment among non-small cell lung cancer patients in China.BMC Public Health. 2025 Jan 15;25(1):164. doi: 10.1186/s12889-024-20951-6. BMC Public Health. 2025. PMID: 39815238 Free PMC article.
-
Optimizing Patient Access to Orphan Medicinal Products: Lessons from Central and Eastern Europe.J Mark Access Health Policy. 2025 May 26;13(2):24. doi: 10.3390/jmahp13020024. eCollection 2025 Jun. J Mark Access Health Policy. 2025. PMID: 40568380 Free PMC article.
-
Navigating barriers to health technology assessment development in Iran: a qualitative exploration of stakeholder perspectives.Arch Public Health. 2025 Mar 27;83(1):83. doi: 10.1186/s13690-025-01566-8. Arch Public Health. 2025. PMID: 40148921 Free PMC article.
References
-
- Németh B, Goettsch W, Kristensen FB, Piniazhko O, Huić M, Tesař T. The transferability of health technology assessment-the European perspective with focus on central and eastern European countries. Expert Rev Pharmacoecon Outcomes Res. (2020) 20:321–30. doi: 10.1080/14737167.2020.1779061, PMID: - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

