Development of Riluzole Analogs with Improved Use-Dependent Inhibition of Skeletal Muscle Sodium Channels
- PMID: 37465302
- PMCID: PMC10350938
- DOI: 10.1021/acsmedchemlett.3c00224
Development of Riluzole Analogs with Improved Use-Dependent Inhibition of Skeletal Muscle Sodium Channels
Abstract
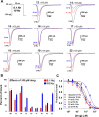
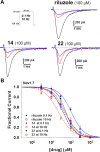
Several commercially available and newly synthesized riluzole analogs were evaluated in vitro as voltage-gated skeletal muscle sodium-channel blockers. Data obtained from the patch-clamp technique demonstrated that potency is well correlated with lipophilicity and the introduction of a protonatable amino function in the benzothiazole 2-position enhances the use-dependent behavior. The most interesting compound, the 2-piperazine analog of riluzole (14), although slightly less potent than the parent compound in the patch-clamp assay as well as in an in vitro model of myotonia, showed greater use-dependent Nav1.4 blocking activity. Docking studies allowed the identification of the key interactions that 14 makes with the amino acids of the local anesthetic binding site within the pore of the channel. The reported results pave the way for the identification of novel compounds useful in the treatment of cell excitability disorders.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

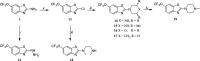
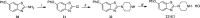




Similar articles
-
Molecular dissection of lubeluzole use-dependent block of voltage-gated sodium channels discloses new therapeutic potentials.Mol Pharmacol. 2013 Feb;83(2):406-15. doi: 10.1124/mol.112.080804. Epub 2012 Nov 21. Mol Pharmacol. 2013. PMID: 23175529
-
Safinamide's potential in treating nondystrophic myotonias: Inhibition of skeletal muscle voltage-gated sodium channels and skeletal muscle hyperexcitability in vitro and in vivo.Exp Neurol. 2020 Jun;328:113287. doi: 10.1016/j.expneurol.2020.113287. Epub 2020 Mar 20. Exp Neurol. 2020. PMID: 32205118
-
Effects of Benzothiazolamines on Voltage-Gated Sodium Channels.Handb Exp Pharmacol. 2018;246:233-250. doi: 10.1007/164_2017_46. Handb Exp Pharmacol. 2018. PMID: 28939972 Review.
-
Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back.Int J Mol Sci. 2023 Jan 3;24(1):857. doi: 10.3390/ijms24010857. Int J Mol Sci. 2023. PMID: 36614292 Free PMC article. Review.
-
Interaction of high concentrations of riluzole with recombinant skeletal muscle sodium channels and adult-type nicotinic receptor channels.Muscle Nerve. 2002 Oct;26(4):539-45. doi: 10.1002/mus.10230. Muscle Nerve. 2002. PMID: 12362421
References
-
- Hays S. J.; Rice M.; Ortwine D. F.; Johnson G.; Schwarz R. D.; Boyd D. K.; Copeland L. F.; Vartanian M. G.; Boxer P. A. Substituted 2-benzothiazolamines as sodium flux inhibitors: quantitative structure-activity relationships and anticonvulsant activity. J. Pharm. Sci. 1994, 83 (10), 1425–1432. 10.1002/jps.2600831013. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Chemical Information

