N-Alkyl Carbamoylimidazoles as Versatile Synthons for the Synthesis of Urea-Based PSMA Inhibitors
- PMID: 37465305
- PMCID: PMC10351058
- DOI: 10.1021/acsmedchemlett.3c00087
N-Alkyl Carbamoylimidazoles as Versatile Synthons for the Synthesis of Urea-Based PSMA Inhibitors
Abstract
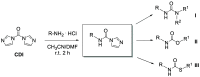
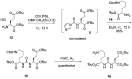
We describe N-alkyl carbamoylimidazoles as readily available and highly versatile synthons for synthesizing urea-based prostate-specific membrane antigen (PSMA) inhibitors. Urea formation proceeded in high yields (>80%) at room temperature under aqueous conditions. All novel compounds were tested for their PSMA inhibitory potency in a cell-based radiometric binding assay. Compound 17 was identified as a novel high-affinity PSMA inhibitor (IC50 = 0.013 μM) suitable for developing an 18F-labeled radioligand for PET imaging of PSMA in prostate cancer.
© 2023 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Bostwick D. G.; Pacelli A.; Blute M.; Roche P.; Murphy G. P. Prostate Specific Membrane Antigen Expression in Prostatic Intraepithelial Neoplasia and Adenocarcinoma: A Study of 184 Cases. Cancer 1998, 82 (11), 2256–2261. 10.1002/(SICI)1097-0142(19980601)82:11<2256::AID-CNCR22>3.0.CO;2-S. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

