Efficacy analysis of empirical bismuth quadruple therapy, high-dose dual therapy, and resistance gene-based triple therapy as a first-line Helicobacter pylori eradication regimen - An open-label, randomized trial
- PMID: 37465346
- PMCID: PMC10350889
- DOI: 10.1515/med-2023-0722
Efficacy analysis of empirical bismuth quadruple therapy, high-dose dual therapy, and resistance gene-based triple therapy as a first-line Helicobacter pylori eradication regimen - An open-label, randomized trial
Abstract
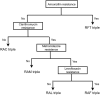
This research aimed to evaluate the eradication efficacy, safety, and gastrointestinal symptom relief rates of empirical bismuth quadruple therapy, high-dose dual therapy, and resistance gene-based triple therapy in primary eradication patients in Yangzhou, China. It also investigated the possible factors influencing the success of different Helicobacter pylori eradication regimens. A single-center, prospective, open-label, randomized controlled study was performed from December 2020 and October 2021, in which 255 patients with H. pylori infection were assigned in a 1:1:1 ratio to the three different groups. Our results showed that high-dose dual therapy (91.0%, 71/78) and resistance gene-based triple therapy (94.9%, 75/79) achieved eradication rates and compliance equivalent to those of empirical bismuth quadruple therapy (85.3%, 64/75) in the per-protocol analysis, while high-dose dual therapy had lower rates of adverse events (11.5%, 9/78, P < 0.05), fewer side effects, and greater safety. Most patients' gastrointestinal discomfort symptoms improved after eradication of H. pylori. Poor compliance (P < 0.05) and antibiotic resistance (P < 0.05) were risk factors for the efficacy of H. pylori eradication. Therefore, the appropriate regimen can be individualized for eradication therapy in clinical practice according to the patient's resistance and tolerance to the drug.
Keywords: Helicobacter pylori; empirical bismuth quadruple therapy; eradication efficacy; high-dose dual therapy; resistance-based triple therapy.
© 2023 the author(s), published by De Gruyter.
Conflict of interest statement
Conflict of interest: All the authors disclose no conflict of interest.
Figures



Similar articles
-
Comparison of high-dose dual therapy with bismuth-containing quadruple therapy in Helicobacter pylori-infected treatment-naive patients: An open-label, multicenter, randomized controlled trial.Pharmacotherapy. 2022 Mar;42(3):224-232. doi: 10.1002/phar.2662. Epub 2022 Feb 5. Pharmacotherapy. 2022. PMID: 35075679 Clinical Trial.
-
Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: a multicentre, open-label, randomised controlled trial.Lancet Gastroenterol Hepatol. 2023 Mar;8(3):228-241. doi: 10.1016/S2468-1253(22)00384-3. Epub 2022 Dec 19. Lancet Gastroenterol Hepatol. 2023. PMID: 36549320 Clinical Trial.
-
High Effective of 14-Day High-Dose PPI- Bismuth-Containing Quadruple Therapy with Probiotics Supplement for Helicobacter Pylori Eradication: A Double Blinded-Randomized Placebo-Controlled Study.Asian Pac J Cancer Prev. 2019 Sep 1;20(9):2859-2864. doi: 10.31557/APJCP.2019.20.9.2859. Asian Pac J Cancer Prev. 2019. PMID: 31554388 Free PMC article. Clinical Trial.
-
Second-line rescue treatment of Helicobacter pylori infection: Where are we now?World J Gastroenterol. 2018 Oct 28;24(40):4548-4553. doi: 10.3748/wjg.v24.i40.4548. World J Gastroenterol. 2018. PMID: 30386104 Free PMC article. Review.
-
High-Dose Dual Therapy Versus Bismuth-Containing Quadruple Therapy for the Treatment of Helicobacter pylori Infection: A Systematic Review with Meta-Analysis.Turk J Gastroenterol. 2022 Jun;33(6):454-462. doi: 10.5152/tjg.2022.21579. Turk J Gastroenterol. 2022. PMID: 35786612 Free PMC article.
References
LinkOut - more resources
Full Text Sources
