Galloylated proanthocyanidins in dentin matrix exhibit biocompatibility and induce differentiation in dental stem cells
- PMID: 37465414
- PMCID: PMC10353770
- DOI: 10.1177/08839115221095154
Galloylated proanthocyanidins in dentin matrix exhibit biocompatibility and induce differentiation in dental stem cells
Abstract
Aim: Grape seed extract contains a complex mixture of proanthocyanidins (PACs), a plant biopolymer used as a biomaterial to improve reparative and preventive dental therapies. Co-polymerization of PACs with type I collagen mechanically reinforces the dentin extracellular matrix. This study assessed the biocompatibility of PACs from grape seed extract on dental pulp stem cells (DPSCs) in a model simulating leaching through dentin to the pulp cavity. The aim was to determine the type of PACs (galloylated vs. non-galloylated) within grape seed extract that are most compatible with dental pulp tissue.
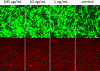
Methodology: Human demineralized dentin was treated with selectively-enriched dimeric PACs prepared from grape seed extract using liquid-liquid chromatography. DPSCs were cultured within a 2D matrix and exposed to PAC-treated dentin extracellular matrix. Cell proliferation was measured using the MTS assay and expression of odontoblastic genes was analyzed by qRT-PCR. Categorization of PACs leaching from dentin was performed using HPLC-MS.
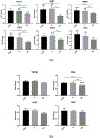
Results: Enriched dimeric fractions containing galloylated PACs increased the expression of certain odontoblastic genes in DPSCs, including Runt-related transcription factor 2 (RUNX2), vascular endothelial growth factor (VEGF), bone morphogenetic protein 2 (BMP2), basic fibroblast growth factor (FGF2), dentin sialophosphoprotein (DSPP) and collagen, type I, alpha 1 (COLI). Galloylated dimeric PACs also exhibited minor effects on DPSC proliferation, resulting in a decrease compared to control after five days of treatment. The non-galloylated dimer fraction had no effect on these genes or on DPSC proliferation.
Conclusions: Galloylated PACs are biocompatible with DPSCs and may exert a beneficial effect on cells within dental pulp tissue. The observed increase in odontoblastic genes induced by galloylated PACs together with a decrease in DPSC proliferation is suggestive of a shift toward cell differentiation. This data supports the use of dimeric PACs as a safe biomaterial, with galloylated dimeric PACs exhibiting potential benefits to odontoblasts supporting dentin regeneration.
Keywords: biocompatibility; cell differentiation; cell viability; dental pulp stem cells; dentin; plant biopolymers; proanthocyanidins.
Conflict of interest statement
Declaration of Conflicting Interests The Authors declare that there is no conflict of interest.
Figures

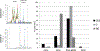
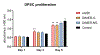
Similar articles
-
Oligomeric proanthocyanidins released from dentin induce regenerative dental pulp cell response.Acta Biomater. 2017 Jun;55:262-270. doi: 10.1016/j.actbio.2017.03.051. Epub 2017 Mar 29. Acta Biomater. 2017. PMID: 28365481 Free PMC article.
-
Demineralized Dentin Matrix Induces Odontoblastic Differentiation of Dental Pulp Stem Cells.Cells Tissues Organs. 2016;201(1):65-76. doi: 10.1159/000440952. Epub 2015 Nov 17. Cells Tissues Organs. 2016. PMID: 26569105
-
Structure-activity relationships of A-and B-type proanthocyanidins in long-term dentin biomodification and biocompatibility.J Dent. 2025 Oct;161:105988. doi: 10.1016/j.jdent.2025.105988. Epub 2025 Jul 20. J Dent. 2025. PMID: 40695440
-
A galloylated dimeric proanthocyanidin from grape seed exhibits dentin biomodification potential.Fitoterapia. 2015 Mar;101:169-78. doi: 10.1016/j.fitote.2014.12.006. Epub 2014 Dec 24. Fitoterapia. 2015. PMID: 25542682 Free PMC article.
-
The biological functions of proanthocyanidin and its application in pig production.Front Vet Sci. 2025 Mar 12;12:1565501. doi: 10.3389/fvets.2025.1565501. eCollection 2025. Front Vet Sci. 2025. PMID: 40144517 Free PMC article. Review.
Cited by
-
Optimization of dental adhesive interfaces using tissue biomodulation with DESIGNER biopolymers.Dent Mater. 2025 Jul;41(7):769-777. doi: 10.1016/j.dental.2025.03.020. Epub 2025 May 2. Dent Mater. 2025. PMID: 40316470
References
-
- Geurtsen W. Biocompatibility of resin-modified filling materials. Crit Rev Oral Biol Med 2000; 11: 333–355. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
