An international consensus definition for contextual factors: findings from a nominal group technique
- PMID: 37465492
- PMCID: PMC10351924
- DOI: 10.3389/fpsyg.2023.1178560
An international consensus definition for contextual factors: findings from a nominal group technique
Abstract
Objective: Emerging literature suggests contextual factors are important components of therapeutic encounters and may substantially influence clinical outcomes of a treatment intervention. At present, a single consensus definition of contextual factors, which is universal across all health-related conditions is lacking. The objective of this study was to create a consensus definition of contextual factors to better refine this concept for clinicians and researchers.
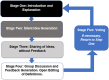
Design: The study used a multi-stage virtual Nominal Group Technique (vNGT) to create and rank contextual factor definitions. Nominal group techniques are a form of consensus-based research, and are beneficial for identifying problems, exploring solutions and establishing priorities.
Setting: International.
Main outcome measures: The initial stages of the vNGT resulted in the creation of 14 independent contextual factor definitions. After a prolonged discussion period, the initial definitions were heavily modified, and 12 final definitions were rank ordered by the vNGT participants from first to last.
Participants: The 10 international vNGT participants had a variety of clinical backgrounds and research specializations and were all specialists in contextual factors research.
Results: A sixth round was used to identify a final consensus, which reflected the complexity of contextual factors and included three primary domains: (1) an overall definition; (2) qualifiers that serve as examples of the key areas of the definition; and (3) how contextual factors may influence clinical outcomes.
Conclusion: Our consensus definition of contextual factors seeks to improve the understanding and communication between clinicians and researchers. These are especially important in recognizing their potential role in moderating and/or mediating clinical outcomes.
Keywords: clinical outcomes; consensus research; contextual factors; nominal group technique; placebo.
Copyright © 2023 Cook, Bailliard, Bent, Bialosky, Carlino, Colloca, Esteves, Newell, Palese, Reed, Vilardaga and Rossettini.
Conflict of interest statement
GR leads education programmes on placebo, nocebo effects and contextual factors in healthcare to under- and post-graduate students along with private CPD courses. CC receives honoraria from book sales and continuing education courses, and is a consultant for Revenite and the Hawkins Foundation. None of the honoraria or consulting work has a competing interest with the work provided in this paper. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Cantrill J. A., Sibbald B., Buetow S. (2011). The Delphi and nominal group techniques in health services research. Int. J. Pharm. Pract. 4, 67–74. doi: 10.1111/j.2042-7174.1996.tb00844.x - DOI
-
- Centers of Disease Control and Prevention . (2012) Principles of Epidemiology, Lesson 1, Section 9. Natural history and Spectrum of disease. Available at: https://www.cdc.gov/csels/dsepd/ss1978/lesson1/section9.html (Accessed December 2022).
Grants and funding
LinkOut - more resources
Full Text Sources
Medical