Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Meta-analysis
- PMID: 37465496
- PMCID: PMC10333252
- DOI: 10.18240/ijo.2023.07.21
Comparison of efficacy of conbercept, aflibercept, and ranibizumab ophthalmic injection in the treatment of macular edema caused by retinal vein occlusion: a Meta-analysis
Abstract
Aim: To evaluate and compare the anatomical and functional outcomes and negative effects of the three anti-vascular endothelial growth factor (VEGF) drugs in the treatment of macular edema (ME) due to retinal vein occlusion (RVO) based on the evidence pooled from current clinical trials and observational studies.
Methods: A systematic literature search was conducted on nine online databases from inception until April 30, 2022. The main endpoints were best corrected visual acuity (BCVA), central macular thickness (CMT), and adverse events (AEs). Cumulative Meta-analysis was conducted to synthesize the outcomes of the drugs. The retrieved data were analyzed using Stata software (version 12.0).
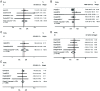
Results: A total of 20 studies comprising 1674 eyes met the inclusion criteria to the Meta-analysis. It was observed that conbercept and aflibercept had better visual acuity effects compared with ranibizumab at 1mo [weight mean difference (WMD)=-0.03, P=0.001; WMD=-0.05, P=0.019], but the effects were not different from that of ranibizumab at 6mo. Moreover, there was not statistically significant difference in the proportion of patients gaining ≥15 letters at 12-24mo between aflibercept and ranibizumab [odds ratio (OR)=1.16, P=0.427]. Conbercept had higher mean CMT change effects at 1mo (WMD=-14.43, P=0.014) and 6mo (WMD=-35.63, P≤0.001) compared with ranibizumab. Meanwhile, the mean CMT change effects at 1mo (WMD=-10.14, P=0.170), 6mo (WMD=-26.98, P=0.140) and 12-24mo (WMD=-12.34, P=0.071) were comparable among the groups. Similarly, AEs were not significantly different among the treatments (OR=0.75, P=0.305; OR=1.04, P=0.89). The stability of effect size of mean BCVA and CMT improved with the increase in sample size. Aflibercept and conbercept required fewer injections compared with ranibizumab.
Conclusion: This is the first study to evaluate the efficacy and AEs of intravitreal administration of conbercept, ranibizumab, and aflibercept in the treatment of RVO-ME. Intravitreal aflibercept or conbercept results in better mean change in vision and CMT reduction compared with ranibizumab. Conbercept can be considered to be a promising and innovative drug with good anti-VEGF effects.
Keywords: aflibercept; anti-vascular endothelial growth factor; conbercept; macular edema; ranibizumab; retinal vein occlusion.
International Journal of Ophthalmology Press.
Figures


Similar articles
-
A systematic review and meta-analysis to compare the efficacy of conbercept with ranibizumab in patients with macular edema secondary to retinal vein occlusion.Medicine (Baltimore). 2020 May 22;99(21):e20222. doi: 10.1097/MD.0000000000020222. Medicine (Baltimore). 2020. PMID: 32481293 Free PMC article.
-
Comparative Efficacy of Pharmacotherapy for Macular Edema Secondary to Retinal Vein Occlusion: A Network Meta-analysis.Front Pharmacol. 2021 Dec 8;12:752048. doi: 10.3389/fphar.2021.752048. eCollection 2021. Front Pharmacol. 2021. PMID: 34955825 Free PMC article.
-
Prospective study of aflibercept for the treatment of persistent macular oedema secondary to retinal vein occlusions in eyes not responsive to long-term treatment with bevacizumab or ranibizumab.Clin Exp Ophthalmol. 2020 Jan;48(1):53-60. doi: 10.1111/ceo.13636. Epub 2019 Oct 10. Clin Exp Ophthalmol. 2020. PMID: 31498950 Clinical Trial.
-
Comparison of conbercept and ranibizumab for the treatment efficacy of diabetic macular edema: a Meta-analysis and systematic review.Int J Ophthalmol. 2019 Sep 18;12(9):1479-1486. doi: 10.18240/ijo.2019.09.17. eCollection 2019. Int J Ophthalmol. 2019. PMID: 31544046 Free PMC article.
-
Real-World Evidence in the Management of Diabetic Macular Edema with Intravitreal Anti-VEGFs in Asia: A Systematic Literature Review.Clin Ophthalmol. 2022 Oct 19;16:3503-3526. doi: 10.2147/OPTH.S378392. eCollection 2022. Clin Ophthalmol. 2022. PMID: 36274678 Free PMC article. Review.
Cited by
-
Effect of simultaneous intravitreal ranibizumab and extended-release dexamethasone injection on patients with naïve versus refractory retinal vein occlusion macular edema: a prospective, multicenter, and interventional open-label study.Int J Ophthalmol. 2025 May 18;18(5):860-867. doi: 10.18240/ijo.2025.05.11. eCollection 2025. Int J Ophthalmol. 2025. PMID: 40385115 Free PMC article.
-
Effectiveness of intravitreal ranibizumab for diabetic macular edema in vitrectomized versus non-vitrectomized eyes: a Meta-analysis.Int J Ophthalmol. 2024 Apr 18;17(4):729-735. doi: 10.18240/ijo.2024.04.18. eCollection 2024. Int J Ophthalmol. 2024. PMID: 38638245 Free PMC article.
-
Prognostic biomarkers in treatment-naïve central retinal vein occlusion with macular edema.Eur J Med Res. 2025 Jul 8;30(1):594. doi: 10.1186/s40001-025-02884-x. Eur J Med Res. 2025. PMID: 40624719 Free PMC article.
-
Clinical effects of atorvastatin combined with conbercept in the treatment of patients with macular edema secondary to retinal vein occlusion and carotid plaque: study protocol for a prospective randomized controlled trial.Trials. 2024 Apr 8;25(1):244. doi: 10.1186/s13063-024-08082-0. Trials. 2024. PMID: 38589960 Free PMC article.
-
Effect of multiple intravitreal injections of conbercept on the cornea in patients with branch retinal vein occlusion-induced macular edema.Front Med (Lausanne). 2025 Jun 25;12:1595543. doi: 10.3389/fmed.2025.1595543. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40636373 Free PMC article.
References
-
- Chen KH, Hsiang EL, Hsu MY, Chou YC, Lin TC, Chang YL, Tsai CY, Li TH, Woung LC, Chen SJ, Peng CH, Hwang DK. Elevation of serum oxidative stress in patients with retina vein occlusions. Acta Ophthalmol. 2019;97(2):e290–e295. - PubMed
-
- Hykin P, Prevost AT, Vasconcelos JC, Murphy C, Kelly J, Ramu J, Hounsome B, Yang Y, Harding SP, Lotery A, Chakravarthy U, Sivaprasad S, LEAVO Study Group Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol. 2019;137(11):1256–1264. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
