Burden of chronic cough in the UK: results from the 2018 National Health and Wellness Survey
- PMID: 37465559
- PMCID: PMC10350679
- DOI: 10.1183/23120541.00157-2023
Burden of chronic cough in the UK: results from the 2018 National Health and Wellness Survey
Abstract
Background: Chronic cough, defined as daily cough for at least 8 weeks, negatively affects quality of life and work productivity and increases healthcare resource utilisation. We aimed to determine the prevalence and burden of chronic cough in the UK.
Methods: Study participants were general population respondents to the 2018 UK National Health and Wellness Survey (NHWS). Respondents completed survey questions relating to health, quality of life, work productivity and activity impairment, and use of healthcare resources. Prevalence estimates were projected to the UK population using post-stratification sampling weights to adjust for sampling bias. The population with chronic cough was matched 1:3 with a group without chronic cough, using propensity score matchingon age, sex and the modified Charlson Comorbidity Index.
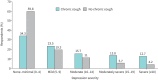
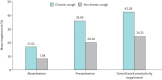
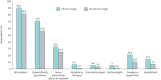
Results: Of 15 000 NHWS respondents, 715 reported chronic cough in the previous 12 months and 918 during their lifetime. Weighted to the UK adult population, the 12-month prevalence of chronic cough was 4.9% and lifetime prevalence was 6.2%. Prevalence of chronic cough was higher among older respondents and those with smoking histories. Chronic cough respondents experienced higher rates of severe anxiety and depression in the past 2 weeks than matched controls. Poor sleep quality and loss of work productivity were also observed. More chronic cough respondents visited a healthcare provider in the past 6 months than respondents without chronic cough with a mean of 5.8 and 3.7 visits per respondent, respectively.
Conclusion: Adults with chronic cough report lower quality of life, reduced work productivity and greater healthcare resource utilisation than matched controls without chronic cough.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: L. McGarvey has received consulting fees from Bayer, Bellus, Merck, Sanofi, Shionogi, Nocion and Chiesi, lecture fees from Glaxo Smith Kline, Merck and Bionorica, and grant support from Merck. Conflict of interest: A.H. Morice has received consulting fees from Bayer, Bellus, Boehringer Ingelheim, Merck, Pfizer, Proctor & Gamble and Shionogi, lecture fees from Boehringer Ingelheim and AstraZeneca, and grant support from Proctor & Gamble, Merck, Afferent and Infirst; and is an associate editor of this journal. Conflict of interest: A. Martin and V.W. Li are employees of Cerner Enviza (formerly Kantar LLC). Conflict of interest: M.J. Doane was an employee of Cerner Enviza (formerly Kantar LLC) during the development and conduct of this study. Conflict of interest: E. Urdaneta, H. Ding and E. Fonseca are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and shareholders in Merck & Co., Inc. Conflict of interest: J. Schelfhout was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and shareholder in Merck & Co., Inc, at the time of the study.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous
