Establishment of a porcine bronchial epithelial cell line and its application to study innate immunity in the respiratory epithelium
- PMID: 37465671
- PMCID: PMC10350646
- DOI: 10.3389/fimmu.2023.1117102
Establishment of a porcine bronchial epithelial cell line and its application to study innate immunity in the respiratory epithelium
Abstract
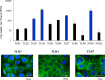
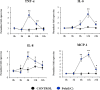
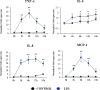
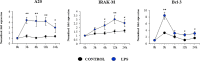
In vitro culture models that precisely mirror the porcine respiratory epithelium are needed to gain insight into how pathogens and host interact. In this study, a new porcine bronchial epithelial cell line, designated as PBE cells, was established from the respiratory tract of a neonatal pig. PBE cells assumed a cobblestone-epithelial like morphology with close contacts between the cells when they reached confluence. The PBE cell line was characterized in terms of its expression of pattern recognition receptors (PRRs) and its ability to respond to the activation of the Toll-like receptor 3 (TLR3) and TLR4 signaling pathways, which are key PRRs involved in the defense of the respiratory epithelium against pathogens. PBE cells stimulated with poly(I:C) were able to up-regulate the expression of IFN-β, IFN-λ1 (IL-29), IFN-λ3 (IL-28B), the antiviral factors Mx1, OAS1, and PKR, as well as the viral PRRs RIG-1 and MDA5. The expression kinetics studies of immune factors in PBE cells allow us to speculate that this cell line can be a useful in vitro tool to investigate treatments that help to potentiate antiviral immunity in the respiratory epithelium of the porcine host. In addition, poly(I:C) and LPS treatments increased the expression of the inflammatory cytokines TNF-α, IL-6, IL-8, and MCP-1/CCL2 and differentially modulated the expression of negative regulators of the TLR signaling pathways. Then, PBE cells may also allow the evaluation of treatments that can regulate TLR3- and TLR4-mediated inflammatory injury in the porcine airway, thereby protecting the host against harmful overresponses.
Keywords: TLR3; TLR4; epithelial cells; porcine respiratory epithelial cell line; respiratory epithelium; viral immunity.
Copyright © 2023 Fukuyama, Zhuang, Toyoshi, Raya Tonetti, Saha, Zhou, Ikeda-Ohtsubo, Nishiyama, Aso, Villena and Kitazawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

