Guizhi Fuling capsule relieves memory deficits by inhibition of microglial neuroinflammation through blocking JAK2/STAT3 pathway in presenilin1/2 conditional double knockout mice
- PMID: 37465679
- PMCID: PMC10350565
- DOI: 10.3389/fimmu.2023.1185570
Guizhi Fuling capsule relieves memory deficits by inhibition of microglial neuroinflammation through blocking JAK2/STAT3 pathway in presenilin1/2 conditional double knockout mice
Abstract
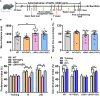
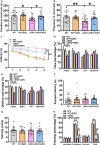
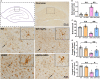
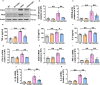
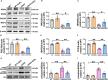
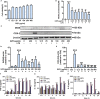
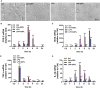
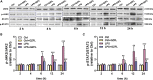
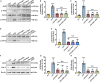
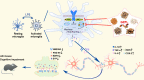
Chronic neuroinflammation has been regarded as an important part of the pathological initiation of Alzheimer's disease (AD), which is associated with the regulation of microglial activation. Preventing microglial activation to inhibit neuroinflammation may become a potential target for the treatment of neurodegenerative diseases. Guizhi Fuling capsule (GZFL) has a strong repression on inflammatory responses. Here, the presenilin1/2 conditional double knockout (PS cDKO) mice, a well-established mouse model of AD, were divided into: WT mice (WT), WT mice+GZFL (WT+GZFL), PS cDKO mice (cDKO), and PS cDKO mice+GZFL (cDKO+GZFL). Mice in the WT+GZFL and cDKO+GZFL group were fed standard chow containing 2000 ppm GZFL for 90 days. After 60 days of GZFL treatment, mice were given to behavioral tests for 30 days in order to explore the effects of GZFL on cognitive and motor function. Then, mice were sacrificed for examining the effects of GZFL on inflammation. Furthermore, primary microglia were obtained from neonatal Sprague-Dawley rats and pretreated with or without GZFL (50 μg/ml) for 1 h in the absence or presence of lipopolysaccharide (LPS) (100 ng/ml) stimulation to speculate whether the underlying mechanism of GZFL's anti-inflammatory potential was closely associated with Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Our findings indicated that GZFL has the ability to alleviate memory deficits in PS cDKO mice, which attributes to the improvement of neuroinflammation by inhibiting microglial activation and the levels of pro-inflammatory mediators. In addition, GZFL could inverse the tau hyperphosphorylation and the lessened expression of synaptic proteins in hippocampus of PS cDKO mice. Furthermore, GZFL prevented LPS-induced neuroinflammatory responses in primary microglia by decreasing the levels of pro-inflammatory mediators. It is noteworthy that therapeutic effects of GZFL on memory impairment are depended on the inhibition of neuroinflammatory responses by the blockage of JAK2/STAT3 signaling pathway. Taken together, GZFL may be an effective compound Chinese medicine for the improvement and postponement of neurodegenerative progression in AD.
Keywords: Alzheimer’s disease; Guizhi Fuling capsule; JAK2/STAT3 pathway; memory deficits; microglial activation; neuroinflammation.
Copyright © 2023 Yang, Tong, Wang, Zhao, Ba, Ahelijiang, Liu, Gao, Zhao, Gu, Yang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

