COVID-19 vaccine induced poor neutralization titers for SARS-CoV-2 omicron variants in maternal and cord blood
- PMID: 37465682
- PMCID: PMC10350671
- DOI: 10.3389/fimmu.2023.1211558
COVID-19 vaccine induced poor neutralization titers for SARS-CoV-2 omicron variants in maternal and cord blood
Abstract
Introduction: Maternally derived antibodies are crucial for neonatal immunity. Understanding the binding and cross-neutralization capacity of maternal and cord antibody responses to SARS-CoV-2 variants following COVID-19 vaccination in pregnancy can inform neonatal immunity.
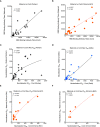
Methods: Here we characterized the binding and neutralizing antibody profile at delivery in 24 pregnant individuals following two doses of Moderna mRNA-1273 or Pfizer BNT162b2 vaccination. We analyzed for SARS-CoV-2 multivariant cross-neutralizing antibody levels for wildtype Wuhan, Delta, Omicron BA1, BA2, and BA4/BA5 variants. In addition, we evaluated the transplacental antibody transfer by profiling maternal and umbilical cord blood.
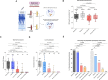
Results: Our results reveal that the current COVID-19 vaccination induced significantly higher RBD-specific binding IgG titers in cord blood compared to maternal blood for both the Wuhan and Omicron BA1 strain. Interestingly, the binding IgG antibody levels for the Omicron BA1 strain were significantly lower when compared to the Wuhan strain in both maternal and cord blood. In contrast to the binding, the Omicron BA1, BA2, and BA4/5 specific neutralizing antibody levels were significantly lower compared to the Wuhan and Delta variants. It is interesting to note that the BA4/5 neutralizing capacity was not detected in either maternal or cord blood.
Discussion: Our data suggest that the initial series of COVID-19 mRNA vaccines were immunogenic in pregnant women, and vaccine-elicited binding antibodies were detectable in cord blood at significantly higher levels for the Wuhan and Delta variants but not for the Omicron variants. Interestingly, the vaccination did not induce neutralizing antibodies for Omicron variants. These results provide novel insight into the impact of vaccination on maternal humoral immune response and transplacental antibody transfer for SARS-CoV-2 variants and support the need for bivalent boosters as new variants emerge.
Keywords: COVID-19 vaccination; cord blood; omicron; pregnancy; variants of concern.
Copyright © 2023 Govindaraj, Cheedarla, Cheedarla, Irby, Neish, Roback, Smith and Velu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Adhikari EH, MacDonald L, SoRelle JA, Morse J, Pruszynski J, Spong CY. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with delta (B.1.617.2) and omicron (B.1.1.529) variant predominance. JAMA (2022) 327(15):1500–2. doi: 10.1001/jama.2022.4356 - DOI - PMC - PubMed
-
- Joseph NT, Dude CM, Verkerke HP, Irby LS, Dunlop AL, Patel RM, et al. Maternal antibody response, neutralizing potency, and placental antibody transfer after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Obstet Gynecol. (2021) 138(2):189–97. doi: 10.1097/AOG.0000000000004440 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

