Tranexamic Acid for Intracerebral Hemorrhage in Patients on Non-Vitamin K Antagonist Oral Anticoagulants (TICH-NOAC): A Multicenter, Randomized, Placebo-Controlled, Phase 2 Trial
- PMID: 37466000
- PMCID: PMC10453353
- DOI: 10.1161/STROKEAHA.123.042866
Tranexamic Acid for Intracerebral Hemorrhage in Patients on Non-Vitamin K Antagonist Oral Anticoagulants (TICH-NOAC): A Multicenter, Randomized, Placebo-Controlled, Phase 2 Trial
Abstract
Background: Evidence-based hemostatic treatment for intracerebral hemorrhage (ICH) associated with non-vitamin K antagonist oral anticoagulants (NOACs) is lacking. Tranexamic acid (TXA) is an antifibrinolytic drug potentially limiting hematoma expansion. We aimed to assess the efficacy and safety of TXA in NOAC-ICH.
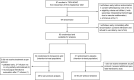
Methods: We performed a double-blind, randomized, placebo-controlled trial at 6 Swiss stroke centers. Patients with NOAC-ICH within 12 hours of symptom onset and 48 hours of last NOAC intake were randomized (1:1) to receive either intravenous TXA (1 g over 10 minutes followed by 1 g over 8 hours) or matching placebo in addition to standard medical care via a centralized Web-based procedure with minimization on key prognostic factors. All participants and investigators were masked to treatment allocation. Primary outcome was hematoma expansion, defined as ≥33% relative or ≥6 mL absolute volume increase at 24 hours and analyzed using logistic regression adjusted for baseline hematoma volume on an intention-to-treat basis.
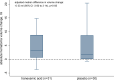
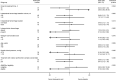
Results: Between December 12, 2016, and September 30, 2021, we randomized 63 patients (median age, 82 years [interquartile range, 76-86]; 40% women; median hematoma volume, 11.5 [4.8-27.4] mL) of the 109 intended sample size before premature trial discontinuation due to exhausted funding. The primary outcome did not differ between TXA (n=32) and placebo (n=31) arms (12 [38%] versus 14 [45%]; adjusted odds ratio, 0.63 [95% CI, 0.22-1.82]; P=0.40). There was a signal for interaction with onset-to-treatment time (Pinteraction=0.024), favoring TXA when administered within 6 hours of symptom onset. Between the TXA and placebo arms, the proportion of participants who died (15 [47%] versus 13 [42%]; adjusted odds ratio, 1.07 [0.37-3.04]; P=0.91) or had major thromboembolic complications within 90 days (4 [13%] versus 2 [6%]; odds ratio, 1.86 [0.37-9.50]; P=0.45) did not differ. All thromboembolic events occurred at least 2 weeks after study treatment, exclusively in participants not restarted on oral anticoagulation.
Conclusions: In a smaller-than-intended NOAC-ICH patient sample, we found no evidence that TXA prevents hematoma expansion, but there were no major safety concerns. Larger trials on hemostatic treatments targeting an early treatment window are needed for NOAC-ICH.
Registration: URL: https://clinicaltrials.gov; Unique identifier: NCT02866838.
Keywords: anticoagulants; cerebral hemorrhage; factor Xa inhibitors; tranexamic acid.
Conflict of interest statement
Figures
References
-
- Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. doi: 10.1136/jnnp-2013-306476 - PubMed
-
- Hald SM, Möller S, García Rodríguez LA, Al-Shahi Salman R, Sharma M, Christensen H, Hellfritzsch M, Pottegård A, Hallas J, Gaist D. Trends in incidence of intracerebral hemorrhage and association with antithrombotic drug use in Denmark, 2005-2018. JAMA network open. 2021;4:e218380–e218380. doi: 10.1001/jamanetworkopen.2021.8380 - PMC - PubMed
-
- Wilson D, Seiffge DJ, Traenka C, Basir G, Purrucker JC, Rizos T, Sobowale OA, Sallinen H, Yeh SJ, Wu TY, et al. ; and the CROMIS-2 Collaborators. Outcome of intracerebral hemorrhage associated with different oral anticoagulants. Neurology. 2017;88:1693–1700. doi: 10.1212/WNL.0000000000003886 - PMC - PubMed
-
- Tsivgoulis G, Wilson D, Katsanos AH, Sargento-Freitas J, Marques-Matos C, Azevedo E, Adachi T, von der Brelie C, Aizawa Y, Abe H, et al. . Neuroimaging and clinical outcomes of oral anticoagulant-associated intracerebral hemorrhage. Ann Neurol. 2018;84:694–704. doi: 10.1002/ana.25342 - PubMed

