Physical Delithiation of Epitaxial LiCoO2 Battery Cathodes as a Platform for Surface Electronic Structure Investigation
- PMID: 37466037
- PMCID: PMC10401565
- DOI: 10.1021/acsami.3c06147
Physical Delithiation of Epitaxial LiCoO2 Battery Cathodes as a Platform for Surface Electronic Structure Investigation
Abstract
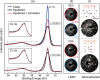
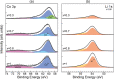
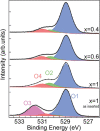
We report a novel delithiation process for epitaxial thin films of LiCoO2(001) cathodes using only physical methods, based on ion sputtering and annealing cycles. Preferential Li sputtering followed by annealing produces a surface layer with a Li molar fraction in the range 0.5 < x < 1, characterized by good crystalline quality. This delithiation procedure allows the unambiguous identification of the effects of Li extraction without chemical byproducts and experimental complications caused by electrolyte interaction with the LiCoO2 surface. An analysis by X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) provides a detailed description of the delithiation process and the role of O and Co atoms in charge compensation. We observe the simultaneous formation of Co4+ ions and of holes localized near O atoms upon Li removal, while the surface shows a (2 × 1) reconstruction. The delithiation method described here can be applied to other crystalline battery elements and provide information on their properties that is otherwise difficult to obtain.
Keywords: absorption spectroscopy; lithium cobalt oxide; lithium ion batteries; photoemission spectroscopy; sputtering.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Janek J.; Zeier W. G. A Solid Future for Battery Development. Nat. Energy 2016, 1, 1614110.1038/nenergy.2016.141. - DOI
-
- Xiao Y.; Wang Y.; Bo S.-H.; Kim J. C.; Miara L. J.; Ceder G. Understanding Interface Stability in Solid-State Batteries. Nat. Rev. Mater. 2020, 5, 105–126. 10.1038/s41578-019-0157-5. - DOI
-
- Lewis J. A.; Tippens J.; Cortes F. J. Q.; McDowell M. T. Chemo-Mechanical Challenges in Solid-State Batteries. Trends Chem. 2019, 1, 845–857. 10.1016/j.trechm.2019.06.013. - DOI
-
- Wang C.; Liang J.; Hwang S.; Li X.; Zhao Y.; Adair K.; Zhao C.; Li X.; Deng S.; Lin X.; Yang X.; Li R.; Huang H.; Zhang L.; Lu S.; Su D.; Sun X. Unveiling the Critical Role of Interfacial Ionic Conductivity in All-Solid-State Lithium Batteries. Nano Energy 2020, 72, 10468610.1016/j.nanoen.2020.104686. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

