Influenza H3 hemagglutinin vaccine with scrambled immunodominant epitopes elicits antibodies directed toward immunosubdominant head epitopes
- PMID: 37466314
- PMCID: PMC10470489
- DOI: 10.1128/mbio.00622-23
Influenza H3 hemagglutinin vaccine with scrambled immunodominant epitopes elicits antibodies directed toward immunosubdominant head epitopes
Abstract
Vaccination is the most effective countermeasure to reduce the severity of influenza. Current seasonal influenza vaccines mainly elicit humoral immunity targeting hemagglutinin (HA). In particular, the amino acid residues around the receptor-binding site in the HA head domain are predominantly targeted by humoral immunity as "immunodominant" epitopes. However, mutations readily accumulate in the head domain due to high plasticity, resulting in antigenic drift and vaccine mismatch, particularly with influenza A (H3N2) viruses. A vaccine strategy that targets more conserved immunosubdominant epitopes is required to attain a universal vaccine. Here, we designed an H3 HA vaccine antigen with various amino acids at immunodominant epitopes of the HA head domain, termed scrambled HA (scrHA). In ferrets, scrHA vaccination induced lower serum neutralizing antibody levels against homologous virus compared with wild-type (WT) HA vaccination; however, similar levels of moderately neutralizing titers against antigenically distinct H3N2 viruses were observed. Ferrets vaccinated with scrHA twice and then challenged with homologous or heterologous virus showed the same level of reduced virus shedding in nasal swabs as WT HA-vaccinated animals but reduced body temperature increase, whereas WT HA-vaccinated ferrets exhibited body temperature increases similar to those of mock-vaccinated animals. scrHA elicited antibodies against HA immunodominant and -subdominant epitopes at lower and higher levels, respectively, than WT HA vaccination, whereas antistalk antibodies were induced at the same level for both groups, suggesting scrHA-induced redirection from immunodominant to immunosubdominant head epitopes. scrHA vaccination thus induced broader coverage than WT HA vaccination by diluting out the immunodominancy of HA head epitopes. IMPORTANCE Current influenza vaccines mainly elicit antibodies that target the immunodominant head domain, where strain-specific mutations rapidly accumulate, resulting in frequent antigenic drift and vaccine mismatch. Targeting conserved immunosubdominant epitopes is essential to attain a universal vaccine. Our findings with the scrHA developed in this study suggest that designing vaccine antigens that "dilute out" the immunodominancy of the head epitopes may be an effective strategy to induce conserved immunosubdominant epitope-based immune responses.
Keywords: ferret; immunosubdominant epitopes; influenza A (H3N2) viruses; universal vaccines.
Conflict of interest statement
Y.K. has received unrelated funding support from Daiichi Sankyo Pharmaceutical, Toyama Chemical, Tauns Laboratories, Inc., Shionogi & Co. LTD, Otsuka Pharmaceutical, KM Biologics, Kyoritsu Seiyaku, Shinya Corporation, and Fuji Rebio. Y.K. and G.N. are co-founders of FluGen.
Figures


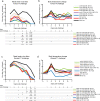

Similar articles
-
Elicitation of Protective Antibodies against 20 Years of Future H3N2 Cocirculating Influenza Virus Variants in Ferrets Preimmune to Historical H3N2 Influenza Viruses.J Virol. 2019 Jan 17;93(3):e00946-18. doi: 10.1128/JVI.00946-18. Print 2019 Feb 1. J Virol. 2019. PMID: 30429350 Free PMC article.
-
Broadly Reactive H2 Hemagglutinin Vaccines Elicit Cross-Reactive Antibodies in Ferrets Preimmune to Seasonal Influenza A Viruses.mSphere. 2021 Mar 10;6(2):e00052-21. doi: 10.1128/mSphere.00052-21. mSphere. 2021. PMID: 33692193 Free PMC article.
-
Evaluation of Next-Generation H3 Influenza Vaccines in Ferrets Pre-Immune to Historical H3N2 Viruses.Front Immunol. 2021 Aug 12;12:707339. doi: 10.3389/fimmu.2021.707339. eCollection 2021. Front Immunol. 2021. PMID: 34475872 Free PMC article.
-
Overcoming Barriers in the Path to a Universal Influenza Virus Vaccine.Cell Host Microbe. 2018 Jul 11;24(1):18-24. doi: 10.1016/j.chom.2018.06.016. Cell Host Microbe. 2018. PMID: 30001520 Review.
-
Broadly neutralizing antibodies to combat influenza virus infection.Antiviral Res. 2024 Jan;221:105785. doi: 10.1016/j.antiviral.2023.105785. Epub 2023 Dec 23. Antiviral Res. 2024. PMID: 38145757 Review.
Cited by
-
Development of broadly protective influenza B vaccines.NPJ Vaccines. 2025 Jan 7;10(1):2. doi: 10.1038/s41541-024-01058-w. NPJ Vaccines. 2025. PMID: 39774170 Free PMC article.
-
Structural Mapping of Polyclonal IgG Responses to HA After Influenza Virus Vaccination or Infection.bioRxiv [Preprint]. 2024 Jul 11:2024.07.08.601940. doi: 10.1101/2024.07.08.601940. bioRxiv. 2024. Update in: mBio. 2025 Mar 12;16(3):e0203024. doi: 10.1128/mbio.02030-24. PMID: 39026813 Free PMC article. Updated. Preprint.
-
Structural mapping of polyclonal IgG responses to HA after influenza virus vaccination or infection.mBio. 2025 Mar 12;16(3):e0203024. doi: 10.1128/mbio.02030-24. Epub 2025 Feb 6. mBio. 2025. PMID: 39912630 Free PMC article.
-
A broad-spectrum vaccine candidate against H5 viruses bearing different sub-clade 2.3.4.4 HA genes.NPJ Vaccines. 2024 Aug 19;9(1):152. doi: 10.1038/s41541-024-00947-4. NPJ Vaccines. 2024. PMID: 39160189 Free PMC article.
-
Single immunization with an influenza hemagglutinin nanoparticle-based vaccine elicits durable protective immunity.Bioeng Transl Med. 2024 Jun 3;9(5):e10689. doi: 10.1002/btm2.10689. eCollection 2024 Sep. Bioeng Transl Med. 2024. PMID: 39553436 Free PMC article.
References
-
- Liu Y, Strohmeier S, González-Domínguez I, Tan J, Simon V, Krammer F, García-Sastre A, Palese P, Sun W. 2021. Mosaic hemagglutinin-based whole Inactivated virus vaccines induce broad protection against influenza B virus challenge in mice. Front Immunol 12:746447. doi:10.3389/fimmu.2021.746447 - DOI - PMC - PubMed
-
- McMahon M, Asthagiri Arunkumar G, Liu W-C, Stadlbauer D, Albrecht RA, Pavot V, Aramouni M, Lambe T, Gilbert SC, Krammer F. 2019. Vaccination with viral vectors expressing chimeric hemagglutinin, NP and M1 antigens protects ferrets against influenza virus challenge. Front Immunol 10:2005. doi:10.3389/fimmu.2019.02005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

