Impact of Molnupiravir Treatment on Patient-Reported COVID-19 Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial
- PMID: 37466374
- PMCID: PMC10686947
- DOI: 10.1093/cid/ciad409
Impact of Molnupiravir Treatment on Patient-Reported COVID-19 Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial
Abstract
Background: Molnupiravir is an orally administered antiviral authorized for COVID-19 treatment in adults at high risk of progression to severe disease. Here, we report secondary and post hoc analyses of participants' self-reported symptoms in the MOVe-OUT trial, which evaluated molnupiravir initiated within 5 days of symptom onset in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed COVID-19.
Methods: Eligible participants completed a 15-item symptom diary daily from day 1 (randomization) through day 29, rating symptom severity as "none," "mild," "moderate," or "severe"; loss of smell and loss of taste were rated as "yes" or "no." Time to sustained symptom resolution/improvement was defined as the number of days from randomization to the first of 3 consecutive days of reduced severity, without subsequent relapse. Time to symptom progression was defined as the number of days from randomization to the first of 2 consecutive days of worsening severity. The Kaplan-Meier method was used to estimate event rates at various time points. The Cox proportional hazards model was used to estimate the hazard ratio between molnupiravir and placebo.
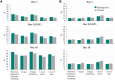
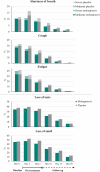
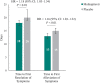
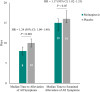
Results: For most targeted COVID-19 symptoms, sustained resolution/improvement was more likely, and progression was less likely, in the molnupiravir versus placebo group through day 29. When evaluating 5 distinctive symptoms of COVID-19, molnupiravir participants had a shorter median time to first resolution (18 vs 20 d) and first alleviation (13 vs 15 d) of symptoms compared with placebo.
Conclusions: Molnupiravir treatment in at-risk, unvaccinated patients resulted in improved clinical outcomes for most participant-reported COVID-19 symptoms compared with placebo. Clinical Trials Registration. ClinicalTrials.gov: NCT04575597.
Keywords: SARS-CoV-2; antiviral; clinical trial; public health; β-D-N4-hydroxycytidine.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. Y. G., A. P., M. G. J., Y. Z., Y. Z., A. W.-D., M. B., A. P., C. D. A., and J. M. N. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA, and may own and/or hold stock options in Merck & Co, Inc, Rahway, NJ, USA. J. D. was an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co, Inc, Rahway, NJ, USA, at the time of study conduct and holds stock and/or stock options in Merck & Co, Inc, Rahway, NJ, USA. A. Puenpatom also reports travel support from Merck & Co., Inc., Rahway, NJ, USA. A. W. holds stock and/or stock options in Carbon Health Technologies, Inc, and CurieAI for advisory work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

