INTRAPERITONEAL CHEMOTHERAPY FOR GASTRIC CANCER WITH PERITONEAL CARCINOMATOSIS: STUDY PROTOCOL OF A PHASE II TRIAL
- PMID: 37466566
- PMCID: PMC10356002
- DOI: 10.1590/0102-672020230026e1744
INTRAPERITONEAL CHEMOTHERAPY FOR GASTRIC CANCER WITH PERITONEAL CARCINOMATOSIS: STUDY PROTOCOL OF A PHASE II TRIAL
Abstract
Background: Peritoneal carcinomatosis in gastric cancer is considered a fatal disease, without expectation of definitive cure. As systemic chemotherapy is not sufficient to contain the disease, a multimodal approach associating intraperitoneal chemotherapy with surgery may represent an alternative for these cases.
Aims: The aim of this study was to investigate the role of intraperitoneal chemotherapy in stage IV gastric cancer patients with peritoneal metastasis.
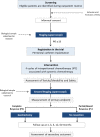
Methods: This study is a single institutional single-arm prospective clinical trial phase II (NCT05541146). Patients with the following inclusion criteria undergo implantation of a peritoneal catheter for intraperitoneal chemotherapy: Stage IV gastric adenocarcinoma; age 18-75 years; Peritoneal carcinomatosis with peritoneal cancer index<12; Eastern Cooperative Oncology Group 0/1; good clinical status; and lab exams within normal limits. The study protocol consists of four cycles of intraperitoneal chemotherapy with paclitaxel associated with systemic chemotherapy. After treatment, patients with peritoneal response assessed by staging laparoscopy undergo conversion gastrectomy.
Results: The primary outcome is the rate of complete peritoneal response. Progression-free and overall survivals are other outcomes evaluated. The study started in July 2022, and patients will be screened for inclusion until 30 are enrolled.
Conclusions: Therapies for advanced gastric cancer patients have been evaluated in clinical trials but without success in patients with peritoneal metastasis. The treatment proposed in this trial can be promising, with easy catheter implantation and ambulatory intraperitoneal chemotherapy regime. Verifying the efficacy and safety of paclitaxel with systemic chemotherapy is an important progress that this study intends to investigate.
RACIONAL:: A carcinomatose peritoneal no câncer gástrico é considerada uma doença fatal, sem expectativa de cura definitiva. Como a quimioterapia sistêmica não é suficiente para conter a doença, uma abordagem multimodal associando a quimioterapia intraperitoneal à cirurgia pode representar uma alternativa para esses casos.
OBJETIVOS:: Investigar o papel da quimioterapia intraperitoneal em pacientes com câncer gástrico estágio IV com metástases peritoneais.
MÉTODOS:: Trata-se de um ensaio clínico prospectivo unicêntrico, braço único, fase II (NCT05541146). Pacientes com os seguintes critérios de inclusão serão submetidos à implantação de cateter peritoneal para quimioterapia intraperitoneal: adenocarcinoma gástrico estágio IV; idade 18-75 anos; carcinomatose peritoneal com índice de câncer peritoneal<12; ECOG 0/1; bom estado clínico e exames laboratoriais dentro da normalidade. O protocolo do estudo consiste em 4 ciclos de quimioterapia intraperitoneal com Paclitaxel associado à quimioterapia sistêmica. Após o tratamento, os pacientes com resposta peritoneal avaliada por laparoscopia serão submetidos à gastrectomia de conversão.
RESULTADOS:: O desfecho primário é a taxa de resposta peritoneal completa. A sobrevida livre de progressão e global são outros desfechos avaliados. O estudo foi iniciado em julho de 2022 e os pacientes serão selecionados para inclusão até que 30 sejam inscritos.
CONCLUSIONS:: Terapias para pacientes com câncer gástrico avançado foram avaliadas em ensaios clínicos, mas sem sucesso em pacientes com metástase peritoneal. O tratamento proposto neste estudo pode ser promissor, com fácil implantação do cateter e regime de quimioterapia intraperitoneal ambulatorial. Verificar a eficácia e segurança do Paclitaxel associado à quimioterapia sistêmica é um progresso importante que o presente estudo pretende investigar.
Conflict of interest statement
Conflicts of interest: None
Figures
References
-
- Badgwell B, Blum M, Das P, Estrella J, Wang X, Ho L, et al. Phase II trial of laparoscopic hyperthermic intraperitoneal chemoperfusion for peritoneal carcinomatosis or positive peritoneal cytology in patients with gastric adenocarcinoma. Ann Surg Oncol. 2017;24(11):3338–3344. doi: 10.1245/s10434-017-6047-4. - DOI - PubMed
-
- Barchi LC, Ramos MFKP, Yagi OK, Mucerino DR, Bresciani CJC, Ribeiro U, Júnior, et al. Brazilian gastric cancer association guidelines (part 1): an update on diagnosis, staging, endoscopic treatment and follow-up. Arq Bras Cir Dig. 2020;33(3):e1535. doi: 10.1590/0102-672020200003e1535. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous